54868-4703 : Ultracet (Apap 325 mg / Tramadol Hydrochloride 37.5 mg) Oral Tablet
| NDC: | 54868-4703 |
| Labeler: | Physicians Total Care, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Ultracet Ultracet |
| Dosage Form: | Oral Tablet, Coated |
| Application #: | NDA021123 |
| Rev. Date: |
Appearance:
| Markings: | O;M;650 |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 15 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
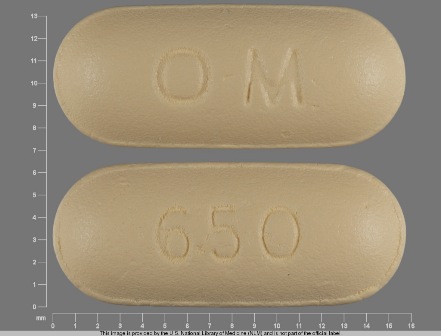
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 54868-4703-1: 10 TABLET, COATED IN 1 BOTTLE (54868‑4703‑1)
- 54868-4703-4: 60 TABLET, COATED IN 1 BOTTLE (54868‑4703‑4)
Active Ingredients:
- Acetaminophen
- Tramadol Hydrochloride
Dosage Strength:
- 325 mg
- 37.5 mg
Inactive Ingredients:
- Powdered Cellulose
- Sodium Starch Glycolate Type a Potato
- Starch, Corn
- Magnesium Stearate
- Hypromelloses
- Polyethylene Glycols
- Polysorbate 80
- Titanium Dioxide
- Ferrosoferric Oxide
- Carnauba Wax
Pharmaceutical Classes:
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
Related Products:
Based on records with the same trade name.- 21695-143 Ultracet (Apap 325 mg / Tramadol Hydrochloride 37.5 mg) Oral Tablet by Rebel Distributors Corp
- 49999-118 Ultracet (Apap 325 mg / Tramadol Hydrochloride 37.5 mg) Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 50090-0748 Ultracet Oral Tablet, Coated by A-s Medication Solutions LLC
- 50458-650 Ultracet (Apap 325 mg / Tramadol Hydrochloride 37.5 mg) Oral Tablet by Janssen Pharmaceuticals, Inc.
- 54569-5308 Ultracet Oral Tablet, Coated by A-s Medication Solutions LLC
- 55289-617 Ultracet (Apap 325 mg / Tramadol Hydrochloride 37.5 mg) Oral Tablet by Pd-rx Pharmaceuticals, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 54868-4700Next: 54868-4705 >
Related Discussions:
Tramadol - Acetaminophen M-O 650
light yellow oblong on the pill it says M-O 650 ## tramadol/acet 37.5 is it addictive? ## The Tablet with the O M 650 ma... 2 replies
light yellow oblong on the pill it says M-O 650 ## tramadol/acet 37.5 is it addictive? ## The Tablet with the O M 650 ma... 2 replies
tramadol and acetaminophen
My husband has artheritis and is taking tramadol twice a day (50mg.) his dr. said he would take one every 4 hrs but he d... 2 replies
My husband has artheritis and is taking tramadol twice a day (50mg.) his dr. said he would take one every 4 hrs but he d... 2 replies
tramadol hcl acetaminophen
red kali 083 ## WHAT IS IT USED FOR? ## This is a nonsteroidal anti-inflammatory, it contains both Acetaminophen and Tra... 2 replies
red kali 083 ## WHAT IS IT USED FOR? ## This is a nonsteroidal anti-inflammatory, it contains both Acetaminophen and Tra... 2 replies
hydrocodone acetaminophen 10 325 and tramadol together
Can I take one hydrocodone acetaminophen 10 325 and one tramadol 50 mg together? One 10 325 does not help much. I do not... 7 replies
Can I take one hydrocodone acetaminophen 10 325 and one tramadol 50 mg together? One 10 325 does not help much. I do not... 7 replies
Hydrocodone Acetaminophen 5 500 Tb and Tramadol
I have Ankylosing Spondylitis. Got Cortisone shots in my lumbar region and Hydrocodone/Acetaminophen 5-500 tb and Tramad... 3 replies
I have Ankylosing Spondylitis. Got Cortisone shots in my lumbar region and Hydrocodone/Acetaminophen 5-500 tb and Tramad... 3 replies
Does Tramadol come in IV form? Is it dangerous? Does it contain aspirin or acetaminophen? (I have hep c and should not take those?)
I have been taking tramadol a long time. For it to work, now I take 7 at a time (my doctor doesn't know this of cour... 2 replies
I have been taking tramadol a long time. For it to work, now I take 7 at a time (my doctor doesn't know this of cour... 2 replies
can these medicines be combine with my daily meds. Tramadol 50mg tablet, and hydrocodone/acetaminophen 5-500 tablet.
Hi; I am taking highblood pressure medicine, Lisnopril-htcz 20/25mg tab and amlodipine Besylate 5mg table. I am taking a... 4 replies
Hi; I am taking highblood pressure medicine, Lisnopril-htcz 20/25mg tab and amlodipine Besylate 5mg table. I am taking a... 4 replies
Ultracet Tab Tramadol Pcn P30
what is the indication or effectiveness to be taken this medication and what is the side effect? ## Ultracet contains th... 1 reply
what is the indication or effectiveness to be taken this medication and what is the side effect? ## Ultracet contains th... 1 reply
TRAMADOL / APAP (ULTRACET) KALI 083
Dark pink tablets (one side has 083 on it the other KALI ) HAS ON BOTTLE Mfg: Par 49884-0946-01 ## No it should not caus... 2 replies
Dark pink tablets (one side has 083 on it the other KALI ) HAS ON BOTTLE Mfg: Par 49884-0946-01 ## No it should not caus... 2 replies
Is Tramadol APAP a generic for Ultracet?
Is Tramadol APAP generic for Ultracet? ## Tramadl/Apap vs. Tramadol is same medicine under different name ? A strong pai... 5 replies
Is Tramadol APAP generic for Ultracet? ## Tramadl/Apap vs. Tramadol is same medicine under different name ? A strong pai... 5 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.