50474-100 : Theo-24 100 mg Extended Release Capsule
| NDC: | 50474-100 |
| Labeler: | Ucb, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Theo-24 Theo-24 |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA087942 |
| Rev. Date: |
Appearance:
| Markings: | Theo;24;100;mg;ucb;2832 |
| Shapes: |
Capsule |
| Colors: |
 Yellow / Yellow /
 Orange Orange |
| Size (mm): | 16 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
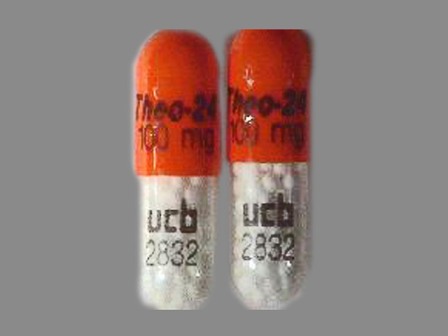
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50474-100-01: 100 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE, PLASTIC (50474‑100‑01)
Active Ingredients:
- Theophylline Anhydrous
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Fd&c Yellow No. 6
- D&c Yellow No. 10
- Fd&c Red No. 40
- Ethylcelluloses
- Gelatin
- Shellac
- Silicon Dioxide
- Sucrose
- Talc
- Titanium Dioxide
Pharmaceutical Classes:
- Methylxanthine [EPC]
- Xanthines [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 50474-200 Theo-24 200 mg Extended Release Capsule by Ucb, Inc.
- 50474-300 Theo-24 300 mg Extended Release Capsule by Ucb, Inc.
- 50474-400 Theo-24 400 mg Extended Release Capsule by Ucb, Inc.
- 52244-100 Theo-24 100 mg Oral Capsule, Extended Release by Actient Pharmaceuticals LLC
- 52244-200 Theo-24 200 mg Oral Capsule, Extended Release by Actient Pharmaceuticals LLC
- 52244-300 Theo-24 300 mg Oral Capsule, Extended Release by Actient Pharmaceuticals LLC
- 52244-400 Theo-24 400 mg Oral Capsule, Extended Release by Actient Pharmaceuticals LLC
- 55154-7206 Theo-24 300 mg Extended Release Capsule by Cardinal Health
- 69189-0300 Theo-24 300 mg Oral Capsule, Extended Release by Avera Mckennan Hospital
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50474-002Next: 50474-150 >
Related Discussions:
Theophylline discontinued
I have been told that theophylline er is being discontinued. Is this true? I have taken it for years for asthma. ## Hell... 244 replies
I have been told that theophylline er is being discontinued. Is this true? I have taken it for years for asthma. ## Hell... 244 replies
Theophylline drug info
Theraputic Class is Xanthine Bronchodilator ## I took Tedral and copirinoal (not sure of the spelling) in liquid forms w... 2 replies
Theraputic Class is Xanthine Bronchodilator ## I took Tedral and copirinoal (not sure of the spelling) in liquid forms w... 2 replies
Theophylline Side Effects
I am wondering about the side affects of the Theophylline? I have asthma, and have been suffering since thanksgiving wit... 1 reply
I am wondering about the side affects of the Theophylline? I have asthma, and have been suffering since thanksgiving wit... 1 reply
theophylline in tea?
Is there theophylline in ALL teas? ## Yes; provided you're drinking actual tea and not an herbal tisane. The tea (Ca... 1 reply
Is there theophylline in ALL teas? ## Yes; provided you're drinking actual tea and not an herbal tisane. The tea (Ca... 1 reply
theophylline-po
small oval white pill on one side theo-dhr 300 half mard on other side ## The pill with imprints theo-dur 300 is Theo-Du... 1 reply
small oval white pill on one side theo-dhr 300 half mard on other side ## The pill with imprints theo-dur 300 is Theo-Du... 1 reply
theophylline sapki
white oblong pill scored in the middle on one side and the other side has pliva459 on it ## This info is from Wikipedia:... 1 reply
white oblong pill scored in the middle on one side and the other side has pliva459 on it ## This info is from Wikipedia:... 1 reply
what the difference between theophylline 100mg sa and er
WHAT IS THE DIFFERENCE BETWEEN THEOPHYLLINE SA AND THEOPHYLLINE ER... ## Hello, Gisela! How are you? There is no differe... 1 reply
WHAT IS THE DIFFERENCE BETWEEN THEOPHYLLINE SA AND THEOPHYLLINE ER... ## Hello, Gisela! How are you? There is no differe... 1 reply
Diphenhydramine Dextromethorophan Caffine Theophylline
Is their any drug, like a cold medicine ect, that would have a combination of caffine, dextromethorophan, diphenhydramin... 1 reply
Is their any drug, like a cold medicine ect, that would have a combination of caffine, dextromethorophan, diphenhydramin... 1 reply
theophyllin
treatment for COPD ## I need information of Theophyllin was is its use and side effects ## I have a chest prob use vente... 3 replies
treatment for COPD ## I need information of Theophyllin was is its use and side effects ## I have a chest prob use vente... 3 replies
Theorides problems
Can I take Adelseril Sibutramina 15 mg. If a have Theorides problems ## TENGO MUCHO TIEMPO CON UN PARPADO INFLAMADO ABES... 2 replies
Can I take Adelseril Sibutramina 15 mg. If a have Theorides problems ## TENGO MUCHO TIEMPO CON UN PARPADO INFLAMADO ABES... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.