50436-0201 : Provigil 200 mg Oral Tablet
| NDC: | 50436-0201 |
| Labeler: | Unit Dose Services |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Provigil Provigil |
| Dosage Form: | Oral Tablet |
| Application #: | NDA020717 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | PROVIGIL;200;MG |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 16 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
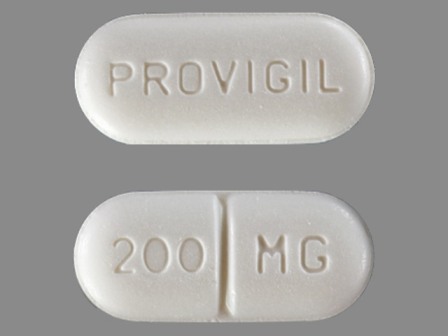
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50436-0201-1: 30 TABLET IN 1 BOTTLE (50436‑0201‑1)
- 50436-0201-2: 60 TABLET IN 1 BOTTLE (50436‑0201‑2)
Active Ingredients:
- Modafinil
Dosage Strength:
- 200 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Povidone
- Starch, Corn
Pharmaceutical Classes:
- Central Nervous System Stimulation [PE]
- Increased Sympathetic Activity [PE]
- Sympathomimetic-like Agent [EPC]
Related Products:
Based on records with the same trade name.- 21695-234 Provigil 100 mg Oral Tablet by Rebel Distributors Corp
- 21695-235 Provigil 200 mg Oral Tablet by Rebel Distributors Corp
- 35356-372 Provigil 200 mg Oral Tablet by Lake Erie Medical Surgical & Supply Dba Quality Care Products LLC
- 54868-4492 Provigil 100 mg Oral Tablet by Physicians Total Care, Inc.
- 54868-4897 Provigil 200 mg Oral Tablet by Physicians Total Care, Inc.
- 55154-0975 Provigil 100 mg Oral Tablet by Cardinal Health
- 63459-101 Provigil 100 mg Oral Tablet by Cephalon, Inc.
- 63459-201 Provigil 200 mg Oral Tablet by Cephalon, Inc.
- 63629-1611 Provigil 200 mg Oral Tablet by Bryant Ranch Prepack
- 63629-3388 Provigil 100 mg Oral Tablet by Bryant Ranch Prepack
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50436-0199Next: 50436-0202 >
Related Discussions:
Provigil (modafinil) for Cough?
Is this used for treating a cough from leukemia? ## You can see the page on Provigil for more information about this med... 4 replies
Is this used for treating a cough from leukemia? ## You can see the page on Provigil for more information about this med... 4 replies
Provigil - Modafinil Dosage
The dosage for this medicine varies from how much low to what? Thank you so much in advance for your time and assistance... 4 replies
The dosage for this medicine varies from how much low to what? Thank you so much in advance for your time and assistance... 4 replies
Modafinil (Provigil) has helped me
I have been using Modafinil (provigil) for a little over a year now, I work a night shift and it's hard for me to ad... 2 replies
I have been using Modafinil (provigil) for a little over a year now, I work a night shift and it's hard for me to ad... 2 replies
modafinil / provigil
Looking for the cost of this drug and is covered under your drug card plan. ## My insurance is ignoring my doctors presc... 2 replies
Looking for the cost of this drug and is covered under your drug card plan. ## My insurance is ignoring my doctors presc... 2 replies
Generic Modafinil, Provigil (brand) or Nuvigil Experiences
Hey all, 1. Anyone tried varying generics of Modafinil? Which ones, and did they work? Anyone tried brand Provigil (supe... 17 replies
Hey all, 1. Anyone tried varying generics of Modafinil? Which ones, and did they work? Anyone tried brand Provigil (supe... 17 replies
Modafinil with alcohol
My specialist wants to prescribe me Modafinil for narcolepsy and I'm doing some research into it and its effects. A ... 3 replies
My specialist wants to prescribe me Modafinil for narcolepsy and I'm doing some research into it and its effects. A ... 3 replies
Modafinil manufacturers
What reputable manufacturers the product Modafinil? I get my prescription from Walmart but I hope it's not being man... 2 replies
What reputable manufacturers the product Modafinil? I get my prescription from Walmart but I hope it's not being man... 2 replies
Modafinil Teva
Is the generic Modafinil produced by Teva gluten-free? It contains pregelantized starch, but I can't find any inform... 2 replies
Is the generic Modafinil produced by Teva gluten-free? It contains pregelantized starch, but I can't find any inform... 2 replies
Modafinil and Viagra Mix. Any Positive Effects?
If I mix Modafinil & Viagra, what can I expect? Any positive results if I have ED? What if I don't have ED &... 1 reply
If I mix Modafinil & Viagra, what can I expect? Any positive results if I have ED? What if I don't have ED &... 1 reply
modafinil and clonazepam and levothyroxine
I have REM sleep disorder, with mild narcolepsy, for which I take low doses of modafinil and clonazepam. I also have Has... 1 reply
I have REM sleep disorder, with mild narcolepsy, for which I take low doses of modafinil and clonazepam. I also have Has... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.