50111-918 : Torsemide 100 mg Oral Tablet
| NDC: | 50111-918 |
| Labeler: | Pliva, Inc |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Torsemide Torsemide |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA076346 |
| Rev. Date: |
Appearance:
| Markings: | PA;918 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 17 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
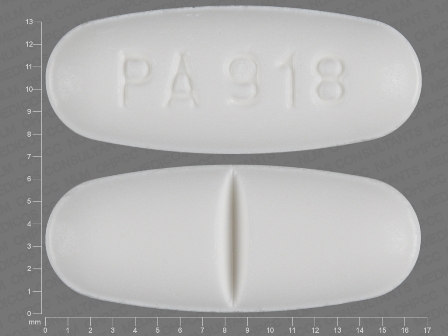
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50111-918-01: 100 TABLET IN 1 BOTTLE (50111‑918‑01)
Active Ingredients:
- Torsemide
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Silicon Dioxide
- Starch, Corn
- Lactose Monohydrate
- Magnesium Stearate
- Sodium Starch Glycolate Type a Potato
Pharmaceutical Classes:
- Increased Diuresis at Loop of Henle [PE]
- Loop Diuretic [EPC]
Related Products:
Based on records with the same trade name.- 50111-915 Torsemide 5 mg Oral Tablet by Pliva, Inc
- 50111-916 Torsemide 10 mg Oral Tablet by Pliva, Inc
- 50111-917 Torsemide 20 mg Oral Tablet by Pliva, Inc
- 0054-0077 Torsemide 20 mg Oral Tablet by Roxane Laboratories, Inc
- 0517-0770 Torsemide 10 mg/ml Intravenous Injection, Solution by American Regent, Inc.
- 0517-0771 Torsemide 10 mg/ml Intravenous Injection, Solution by American Regent, Inc.
- 0603-6134 Torsemide 5 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-6135 Torsemide 10 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-6136 Torsemide 20 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-6137 Torsemide 100 mg Oral Tablet by Qualitest Pharmaceuticals
- 0615-5575 Torsemide 20 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-7997 Torsemide 20 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0904-7283 Torsemide 20 mg Oral Tablet by Major Pharmaceuticals
- 23155-871 Torsemide 5 mg Oral Tablet by Heritage Pharmaceuticals Inc. D/B/A Avet Pharmaceuticals Inc.
- 23155-872 Torsemide 10 mg Oral Tablet by Heritage Pharmaceuticals Inc. D/B/A Avet Pharmaceuticals Inc.
- 23155-873 Torsemide 20 mg Oral Tablet by Heritage Pharmaceuticals Inc. D/B/A Avet Pharmaceuticals Inc.
- 23155-874 Torsemide 100 mg Oral Tablet by Heritage Pharmaceuticals Inc. D/B/A Avet Pharmaceuticals Inc.
- 31722-529 Torsemide 5 mg Oral Tablet by Camber Pharmaceuticals, Inc.
- 31722-530 Torsemide 10 mg Oral Tablet by Camber Pharmaceuticals, Inc.
- 31722-531 Torsemide 20 mg Oral Tablet by Camber Pharmaceuticals, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50111-917Next: 50111-990 >
Related Discussions:
TORSEMIDE 20MG
TAB WHITE ROUND SCORED ON ONE SIDE PA 917 ON OTHER SIDE ## Based on the description provided, I found your pill to be To... 3 replies
TAB WHITE ROUND SCORED ON ONE SIDE PA 917 ON OTHER SIDE ## Based on the description provided, I found your pill to be To... 3 replies
torsemide and spironolactone
What is the differants can you take both at the same time ## Hello, Theresa! How are you? They are both used to help rem... 1 reply
What is the differants can you take both at the same time ## Hello, Theresa! How are you? They are both used to help rem... 1 reply
Torsemide Tab 20mg/Leg cramps
Would like to know if you get leg cramps following taking Torsimide, Also was told that Fluid pills, namely Lasix is dan... 1 reply
Would like to know if you get leg cramps following taking Torsimide, Also was told that Fluid pills, namely Lasix is dan... 1 reply
Torsemide Tab 20mg
What causes the ankles to swell so much and I feel like my chest, is jiggling with fluid ## Have you consulted a doctor?... 1 reply
What causes the ankles to swell so much and I feel like my chest, is jiggling with fluid ## Have you consulted a doctor?... 1 reply
Poor response to torsemide
My husband has chf so takes this med. It hasn't worked after taking double dose. He weight is increasing..ten lbs si... 1 reply
My husband has chf so takes this med. It hasn't worked after taking double dose. He weight is increasing..ten lbs si... 1 reply
torsemide
what is this med ?...
what is this med ?...
Tab. Torsemide plus
Torsemide plus not available in kolkata of India. Doctor advice me to take this medicine. What can I do? Please tell me ...
Torsemide plus not available in kolkata of India. Doctor advice me to take this medicine. What can I do? Please tell me ...
Who launched Torsemide, Amlodipine and telmisartan for the first time in India and when?
Exact year and the company name that launched the generic version. ## Who launched telmisartan and amlodipine combinatio... 1 reply
Exact year and the company name that launched the generic version. ## Who launched telmisartan and amlodipine combinatio... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.