49349-933 : Benztropine Mesylate 1 mg Oral Tablet
| NDC: | 49349-933 |
| Labeler: | Remedyrepack Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Benztropine Mesylate Benztropine Mesylate |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA040742 |
| Rev. Date: |
Appearance:
| Markings: | 2326;V |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 10 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
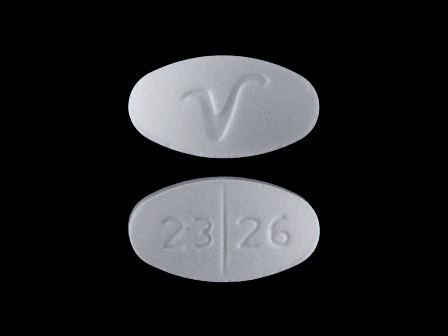
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 49349-933-02: 30 TABLET IN 1 BLISTER PACK (49349‑933‑02)
- 49349-933-10: 6 TABLET IN 1 BLISTER PACK (49349‑933‑10)
Active Ingredients:
- Benztropine Mesylate
Dosage Strength:
- 1 mg
Inactive Ingredients:
- Croscarmellose Sodium
- Anhydrous Dibasic Calcium Phosphate
- Lactose Monohydrate
- Magnesium Stearate
- Cellulose, Microcrystalline
Pharmaceutical Classes:
- Anticholinergic [EPC]
- Antihistamine [EPC]
- Cholinergic Antagonists [MoA]
- Histamine Receptor Antagonists [MoA]
Related Products:
Based on records with the same trade name.- 49349-126 Benztropine Mesylate 500 Mcg Oral Tablet by Remedyrepack Inc.
- 49349-231 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 49349-461 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 49349-519 Benztropine Mesylate 500 Mcg Oral Tablet by Remedyrepack Inc.
- 49349-585 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 49349-656 Benztropine Mesylate 500 Mcg Oral Tablet by Remedyrepack Inc.
- 49349-664 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 49349-673 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 49349-700 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 24236-015 Benztropine Mesylate 500 Mcg Oral Tablet by Remedyrepack Inc.
- 24236-705 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 52125-073 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 52125-169 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 52125-330 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 52125-431 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 52125-869 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 61786-008 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- 61786-013 Benztropine Mesylate .5 mg Oral Tablet by Remedyrepack Inc.
- 61786-018 Benztropine Mesylate 2 mg Oral Tablet by Remedyrepack Inc.
- 61786-038 Benztropine Mesylate 1 mg Oral Tablet by Remedyrepack Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 49349-932Next: 49349-934 >
Related Discussions:
benztropine effects
Will this drug make you feel like you're spaced out as a side effect, or act like a benzo? Or is it like thorazine, ... 4 replies
Will this drug make you feel like you're spaced out as a side effect, or act like a benzo? Or is it like thorazine, ... 4 replies
Benztropine mesylate
## In regards to Benztropine mesylate... More details about this medication are available at: If you have any more quest... 2 replies
## In regards to Benztropine mesylate... More details about this medication are available at: If you have any more quest... 2 replies
Is Benztropine the same as Valium?
Is benztropine medicine considered a valium? Because the pills that i have, have the imprint of 23 26 v ## Hello, Tony! ... 2 replies
Is benztropine medicine considered a valium? Because the pills that i have, have the imprint of 23 26 v ## Hello, Tony! ... 2 replies
Is Benztropine a narcotic?
Is benztropine a narcotic and does it cause blurry vision? I am trying to get off all narcotics given to me for pain and... 1 reply
Is benztropine a narcotic and does it cause blurry vision? I am trying to get off all narcotics given to me for pain and... 1 reply
How To Stop Taking Benztropine and risperidal without withdrals
Taking one in the morning along with two risperidal and one in the evening with two risperidal ## If you want to get off... 2 replies
Taking one in the morning along with two risperidal and one in the evening with two risperidal ## If you want to get off... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.