43063-046 : Chlordiazepoxide Hydrochloride 25 mg Oral Capsule
| NDC: | 43063-046 |
| Labeler: | Pd-rx Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Chlordiazepoxide Hydrochloride Chlordiazepoxide Hydrochloride |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA084769 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | barr;159 |
| Shapes: |
Capsule |
| Colors: |
 Green / Green /
 White White |
| Size (mm): | 14 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
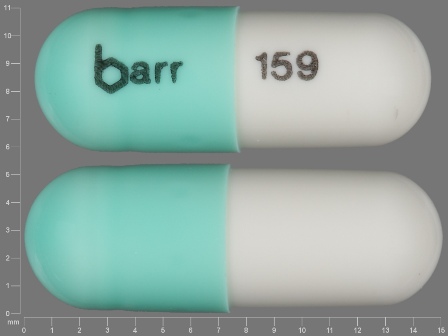
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 43063-046-03: 3 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑03)
- 43063-046-06: 6 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑06)
- 43063-046-12: 12 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑12)
- 43063-046-15: 15 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑15)
- 43063-046-19: 19 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑19)
- 43063-046-24: 24 CAPSULE IN 1 BOTTLE, PLASTIC (43063‑046‑24)
Active Ingredients:
- Chlordiazepoxide Hydrochloride
Dosage Strength:
- 25 mg
Inactive Ingredients:
- D&c Yellow No. 10
- Fd&c Blue No. 1
- Aluminum Oxide
- Gelatin
- Anhydrous Lactose
- Cellulose, Microcrystalline
- Shellac
- Titanium Dioxide
- Fd&c Blue No. 2
- Fd&c Red No. 40
- Propylene Glycol
- Ferrosoferric Oxide
Pharmaceutical Classes:
- Benzodiazepine [EPC]
- Benzodiazepines [CS]
Related Products:
Based on records with the same trade name.- 0555-0033 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule by Barr Laboratories Inc.
- 0555-0158 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule by Barr Laboratories Inc.
- 0555-0159 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule by Barr Laboratories Inc.
- 17856-0164 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule by Atlantic Biologicals Corp.
- 24236-040 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule by Remedyrepack Inc.
- 24236-517 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule by Remedyrepack Inc.
- 24236-800 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule by Remedyrepack Inc.
- 42291-210 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule by Avkare, Inc.
- 42291-211 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule by Avkare, Inc.
- 42291-212 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule by Avkare, Inc.
- 42806-561 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 42806-562 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 42806-563 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 42806-564 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 42806-565 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 42806-566 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule, Gelatin Coated by Epic Pharma LLC
- 43353-699 Chlordiazepoxide Hydrochloride 25 mg Oral Capsule, Gelatin Coated by Aphena Pharma Solutions - Tennessee, LLC
- 43353-969 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule, Gelatin Coated by Aphena Pharma Solutions - Tennessee, LLC
- 43547-251 Chlordiazepoxide Hydrochloride 5 mg Oral Capsule by Solco Healthcare Us LLC
- 43547-252 Chlordiazepoxide Hydrochloride 10 mg Oral Capsule by Solco Healthcare Us LLC
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 43063-045Next: 43063-047 >
Related Discussions:
Chlordiazepoxide-clidinium substitute
Mr doctor wrote prescription for Librax and did not specify brand name only. My local pharmacy dispensed Chlordiazepoxid... 8 replies
Mr doctor wrote prescription for Librax and did not specify brand name only. My local pharmacy dispensed Chlordiazepoxid... 8 replies
Chlordiazepoxide HCl and Clidinium Bromide Capsules
Green cap A-018 ## Just to comfirm, the pill in description is Chlordiazepoxide + Clidinium (5 mg - 2.5 mg). To view inf... 5 replies
Green cap A-018 ## Just to comfirm, the pill in description is Chlordiazepoxide + Clidinium (5 mg - 2.5 mg). To view inf... 5 replies
Chlordiazepoxide + Clidinium Information
It is Generic for Librax®, (Chlordiazepoxide HCl + Clidinium HBr). It's Strength is 5mg/2.5mg. It is a combinat... 5 replies
It is Generic for Librax®, (Chlordiazepoxide HCl + Clidinium HBr). It's Strength is 5mg/2.5mg. It is a combinat... 5 replies
Chlordiazepoxide Clidinium Caps Side Effects
chlordiazepoxide clidinium caps can these affect your blood pressure ## Some adverse reactions reported during therapy w... 3 replies
chlordiazepoxide clidinium caps can these affect your blood pressure ## Some adverse reactions reported during therapy w... 3 replies
Chlordiazepoxide Clidinium Capsule RE369
I have always filled this prescription and gotten small green capsules. Today I received yellow capsules with RE369 on t... 3 replies
I have always filled this prescription and gotten small green capsules. Today I received yellow capsules with RE369 on t... 3 replies
Chlordiazepoxide affecting libido
Took 25mg tabs of librium for a week. Noticed that my libido disappeared. So i stopped taking it. How long will it take ... 1 reply
Took 25mg tabs of librium for a week. Noticed that my libido disappeared. So i stopped taking it. How long will it take ... 1 reply
Chlordiazepoxide/Clidinium caps
Yes, it's ridiculous that both medicare and united health care will not pay for this! Or the generic librax. I have ... 1 reply
Yes, it's ridiculous that both medicare and united health care will not pay for this! Or the generic librax. I have ... 1 reply
Chlordiazepoxide-Clidinium 5 mg-2.5 mg
Does this medication have any interactions with the following drugs: Lamictal, Lithium Carbonate, Glucophage, Buspirone?... 1 reply
Does this medication have any interactions with the following drugs: Lamictal, Lithium Carbonate, Glucophage, Buspirone?... 1 reply
Chlordiazepoxide yellow capsule
re389 ## Yes, these are made by a pharmaceutical company called Rivers Edge, this the RE 369 on the capsule. Chlordiazep... 1 reply
re389 ## Yes, these are made by a pharmaceutical company called Rivers Edge, this the RE 369 on the capsule. Chlordiazep... 1 reply
Generic For Librax-chlordiazepoxide clidinium
I am trying to get a prescription program. I am on medicare. None of the prescrption programs cover the drug listed abov... 1 reply
I am trying to get a prescription program. I am on medicare. None of the prescrption programs cover the drug listed abov... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.