21695-665 : Plavix 75 mg Oral Tablet
| NDC: | 21695-665 |
| Labeler: | Rebel Distributors Corp |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Plavix Plavix |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA020839 |
Appearance:
| Markings: | 75;1171 |
| Shapes: |
Round |
| Colors: |
 Pink Pink |
| Size (mm): | 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
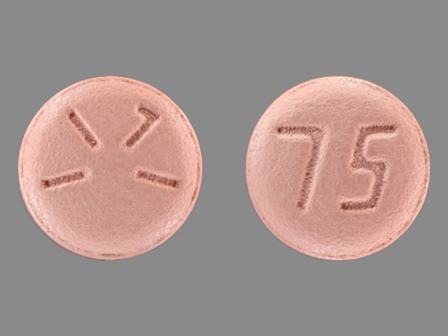
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 21695-665-30: 30 TABLET, FILM COATED IN 1 BOTTLE (21695‑665‑30)
- 21695-665-90: 90 TABLET, FILM COATED IN 1 BOTTLE (21695‑665‑90)
Active Ingredients:
- Clopidogrel Bisulfate
Dosage Strength:
- 75 mg
Inactive Ingredients:
- Castor Oil
- Hydroxypropyl Cellulose
- Mannitol
- Cellulose, Microcrystalline
- Polyethylene Glycol 6000
- Ferric Oxide Red
- Lactose Monohydrate
- Titanium Dioxide
- Triacetin
- Carnauba Wax
Pharmaceutical Classes:
- Decreased Platelet Aggregation [PE]
- P2Y12 Platelet Inhibitor [EPC]
- P2Y12 Receptor Antagonists [MoA]
- Cytochrome P450 2C8 Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0024-1171 Plavix 75 mg Oral Tablet, Film Coated by Sanofi-aventis U.S. LLC
- 0024-1332 Plavix 300 mg Oral Tablet, Film Coated by Sanofi-aventis U.S. LLC
- 16590-288 Plavix 75 mg Oral Tablet by Stat Rx USA LLC
- 24236-824 Plavix 75 mg Oral Tablet by Remedyrepack Inc.
- 49999-402 Plavix 75 mg Oral Tablet by Lake Erie Medical & Surgical Supply Dba Quality Care Products LLC
- 53808-0763 Plavix 75 mg Oral Tablet by State of Florida Doh Central Pharmacy
- 54569-4700 Plavix 75 mg Oral Tablet by A-s Medication Solutions LLC
- 54868-4070 Plavix 75 mg Oral Tablet by Physicians Total Care, Inc.
- 55154-2016 Plavix 75 mg Oral Tablet by Cardinal Health
- 55154-2022 Plavix 300 mg Oral Tablet by Cardinal Health
- 55289-911 Plavix 75 mg Oral Tablet by Pd-rx Pharmaceuticals, Inc.
- 63629-1598 Plavix 75 mg Oral Tablet by Bryant Ranch Prepack
- 63653-1171 Plavix 75 mg Oral Tablet by Bristol-myers Squibb/Sanofi Pharmaceuticals Partnership
- 63653-1332 Plavix 300 mg Oral Tablet by Bristol-myers Squibb/Sanofi Pharmaceuticals Partnership
- 67046-604 Plavix 75 mg Oral Tablet by Contract Pharmacy Services-pa
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 21695-664Next: 21695-666 >
Related Discussions:
Discontinuing my use of Plavix (Clopidogrel)
I am 62 and have no history of hypertension, yet there was a sudden spike in my blood pressure reaching 160/100, so my D... 2 replies
I am 62 and have no history of hypertension, yet there was a sudden spike in my blood pressure reaching 160/100, so my D... 2 replies
Withdrawal Symptoms from Plavix Clopidogrel
I have been on Clopidogrel 75mg after having a slight heart attack thought to have been caused by a blood clot in a smal... 6 replies
I have been on Clopidogrel 75mg after having a slight heart attack thought to have been caused by a blood clot in a smal... 6 replies
Clopidogrel And Atorvastatin
I've been on 75mg Clopidogrel daily for the past month. My hair is falling out at an alarming rate, losing appetite/... 8 replies
I've been on 75mg Clopidogrel daily for the past month. My hair is falling out at an alarming rate, losing appetite/... 8 replies
Clopidogrel tab 75mg
## what kind of sickness is clopidogrel good for ## my mom got clopidogrel to take, the doc say she have a heart problem... 5 replies
## what kind of sickness is clopidogrel good for ## my mom got clopidogrel to take, the doc say she have a heart problem... 5 replies
Clopidogrel Drug Information
What is the mechanism of action, therapeutic benefits, side effects and any precautions that should be taken with Clopid... 4 replies
What is the mechanism of action, therapeutic benefits, side effects and any precautions that should be taken with Clopid... 4 replies
Clopidogrel - temporary stoppage before cataract surgery
Hi. I'm 75 years old. My cardiologist prescribed Deplatt Cv (Ecosprin 75 mg, Atorvastatin 10 mg, and Clopidogrel 75 ... 2 replies
Hi. I'm 75 years old. My cardiologist prescribed Deplatt Cv (Ecosprin 75 mg, Atorvastatin 10 mg, and Clopidogrel 75 ... 2 replies
clopidogrel,cramps
I have been on clopidogrel for 8 years after insertion of a stent. During this period I suffered the most painful crampi... 2 replies
I have been on clopidogrel for 8 years after insertion of a stent. During this period I suffered the most painful crampi... 2 replies
clopidogrel tablets i p
I WANT TO KNOW CLOPIDOGREL TAB IP and CLOPIDOGREL TABLET (with out IP) are same. ## dear sir I am using clopacin 75mg fr... 2 replies
I WANT TO KNOW CLOPIDOGREL TAB IP and CLOPIDOGREL TABLET (with out IP) are same. ## dear sir I am using clopacin 75mg fr... 2 replies
Clopidogrel and bruising
I have been taking Clopidogrel for 3 years and the bruising I get has gradually gotten worse. It appears all over my bod... 1 reply
I have been taking Clopidogrel for 3 years and the bruising I get has gradually gotten worse. It appears all over my bod... 1 reply
Clopidogrel & Depression - Any Connection?
Hi Everyone... Has anyone experienced depression while on 75mg of Clopidogrel daily? Does anyone know, if there are any ... 1 reply
Hi Everyone... Has anyone experienced depression while on 75mg of Clopidogrel daily? Does anyone know, if there are any ... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.