0173-0760 : Lamictal XR Orange Patient Titration Kit
| NDC: | 0173-0760 |
| Labeler: | Glaxosmithkline LLC |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Lamictal XR Lamictal XR |
| Dosage Form: | Kit |
| Application #: | NDA022115 |
| Rev. Date: |
Appearance:
| Markings: | LAMICTAL;XR;100 LAMICTAL;XR;25 LAMICTAL;XR;50 |
| Shapes: |
Round |
| Colors: |
 Green / Green /
 White White Yellow / Yellow /
 White White Orange / Orange /
 White White |
| Size (mm): | 10 9 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
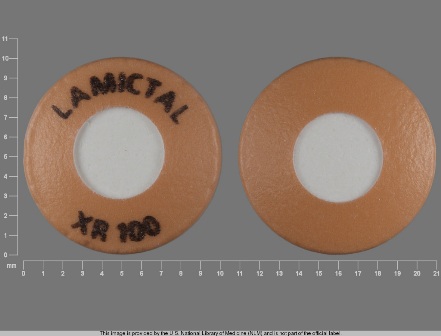


The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0173-0760-00: 1 BLISTER PACK IN 1 PACKAGE, COMBINATION (0173‑0760‑00) > 1 KIT IN 1 BLISTER PACK
- 0173-0760-60: 1 BLISTER PACK IN 1 PACKAGE, COMBINATION (0173‑0760‑60) > 1 KIT IN 1 BLISTER PACK
Active Ingredients:
- Lamotrigine
Kit Contents:
- Lamictal
- Lamitcal
Inactive Ingredients:
- Glyceryl Monostearate
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Methacrylic Acid
- Polyethylene Glycol 400
- Polysorbate 80
- Silicon Dioxide
- Titanium Dioxide
- Triethyl Citrate
- Ferrosoferric Oxide
- Ferric Oxide Yellow
- Glyceryl Monostearate
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Methacrylic Acid
- Polyethylene Glycol 400
- Polysorbate 80
- Titanium Dioxide
- Triethyl Citrate
- Ferric Oxide Yellow
- Ferric Oxide Red
- Glyceryl Monostearate
- Hypromelloses
- Lactose Monohydrate
- Magnesium Stearate
- Methacrylic Acid
- Polyethylene Glycol 400
- Polysorbate 80
- Titanium Dioxide
- Triethyl Citrate
- Silicon Dioxide
- Ferric Oxide Yellow
Related Products:
Based on records with the same trade name.- 0173-0526 Lamictal Cd 5 mg Chewable Tablet by Glaxosmithkline LLC
- 0173-0527 Lamictal Cd 25 mg Chewable Tablet by Glaxosmithkline LLC
- 0173-0594 Lamictal Orange (For Patients Not Taking Carbamazepine, Phenytoin, Phenobarbital, Primidone, Or Rifampin and Not Taking Valproate) by Glaxosmithkline LLC
- 0173-0633 Lamictal Blue (For Patients Taking Valproate) by Glaxosmithkline LLC
- 0173-0642 Lamictal 100 mg Oral Tablet by Glaxosmithkline LLC
- 0173-0643 Lamictal 150 mg Oral Tablet by Glaxosmithkline LLC
- 0173-0644 Lamictal 200 mg Oral Tablet by Glaxosmithkline LLC
- 0173-0699 Lamictal 2 mg Chewable Tablet by Glaxosmithkline LLC
- 0173-0754 Lamictal XR 25 mg 24 Hr Extended Release Tablet by Glaxosmithkline LLC
- 0173-0755 Lamictal XR 50 mg 24 Hr Extended Release Tablet by Glaxosmithkline LLC
- 0173-0756 Lamictal XR 100 mg 24 Hr Extended Release Tablet by Glaxosmithkline LLC
- 0173-0757 Lamictal XR 200 mg 24 Hr Extended Release Tablet by Glaxosmithkline LLC
- 0173-0758 Lamictal XR Blue Patient Titration Kit (For Patients Taking Valproate) by Glaxosmithkline LLC
- 0173-0759 Lamictal XR Green Patient Titration Kit (For Patients Taking Carbamazepine, Phenytoin, Phenobarbital, Or Primidone, and Not Taking Valproate) by Glaxosmithkline LLC
- 0173-0761 24 Hr Lamictal 300 mg Extended Release Enteric Coated Tablet by Glaxosmithkline LLC
- 0173-0772 Lamictal Odt 25 mg Disintegrating Tablet by Glaxosmithkline LLC
- 0173-0774 Lamictal Odt 50 mg Disintegrating Tablet by Glaxosmithkline LLC
- 0173-0776 Lamictal Odt 100 mg Disintegrating Tablet by Glaxosmithkline LLC
- 0173-0777 Lamictal Odt 200 mg Disintegrating Tablet by Glaxosmithkline LLC
- 0173-0778 Lamictal Odt Orange Patient Titration Kit (For Patients Not Taking Enzyme-inducing Drugs Or Valproate) by Glaxosmithkline LLC
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0173-0759Next: 0173-0761 >
Related Discussions:
Lamotrigine 200 mg tab by northstar
Been on generic by Northstar for over a year then this last June 2013 I filled my rx again and within 48 hours I went th... 8 replies
Been on generic by Northstar for over a year then this last June 2013 I filled my rx again and within 48 hours I went th... 8 replies
Lamotrigine Drug Information
Would like to know what this med is used for?? ## triangular shape lamotrigine with 93 on front and 463 on back - How ma... 7 replies
Would like to know what this med is used for?? ## triangular shape lamotrigine with 93 on front and 463 on back - How ma... 7 replies
lamotrigine cough and vomiting
I have bipolar disorder and have been on almost everything for it, was taking depakote and Saphris which worked great bu... 3 replies
I have bipolar disorder and have been on almost everything for it, was taking depakote and Saphris which worked great bu... 3 replies
lamotrigine and vistaril
My girlfriend just got prescribed these 2 meds together for bipolar disorder. Is this safe? The vistaril is 50mg twice a... 2 replies
My girlfriend just got prescribed these 2 meds together for bipolar disorder. Is this safe? The vistaril is 50mg twice a... 2 replies
lamotrigine 100 mg
round white tablet imprint zc80 ## I want to verify that the pills in this bottle are Lamotrigine 100 Mg. The pills are ... 2 replies
round white tablet imprint zc80 ## I want to verify that the pills in this bottle are Lamotrigine 100 Mg. The pills are ... 2 replies
Lamotrigine side effects
I have been taking this medicine for the last 9 months. Whenever my dose is increased above 100mg i am facing body aches... 2 replies
I have been taking this medicine for the last 9 months. Whenever my dose is increased above 100mg i am facing body aches... 2 replies
Lamotrigine split dose
Can Lamotrigine 150 be cut in quarters to lower the dose? I have many 150mg left and would like to use them up if I can ... 1 reply
Can Lamotrigine 150 be cut in quarters to lower the dose? I have many 150mg left and would like to use them up if I can ... 1 reply
Lamotrigine - feelings of insecurity and despair
Pending a formal diagnosis (one psychiatrist felt I was low-level bipolar, my current psychiatrist feels I have a severe... 1 reply
Pending a formal diagnosis (one psychiatrist felt I was low-level bipolar, my current psychiatrist feels I have a severe... 1 reply
lamotrigine is this an opioid
I need to know if this medication is an opioid ## Hello, Twiggy! How are you? No, the FDA classifies Lamotrigine as an a... 1 reply
I need to know if this medication is an opioid ## Hello, Twiggy! How are you? No, the FDA classifies Lamotrigine as an a... 1 reply
lamotrigine 100mg
pink 463 ## Does it also have a 93 on it or the word TEVA? The newer tablets will have Teva on it, since they are gettin... 1 reply
pink 463 ## Does it also have a 93 on it or the word TEVA? The newer tablets will have Teva on it, since they are gettin... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.