0172-7311 : Cyclosporine, Modified 50 mg Oral Capsule
| NDC: | 0172-7311 |
| Labeler: | Ivax Pharmaceuticals, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Cyclosporine Cyclosporine |
| Dosage Form: | Oral Capsule, Liquid Filled |
| Application #: | ANDA065110 |
| Rev. Date: |
Appearance:
| Markings: | 50;mg |
| Shapes: |
Oval |
| Colors: |
 Yellow Yellow |
| Size (mm): | 21 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
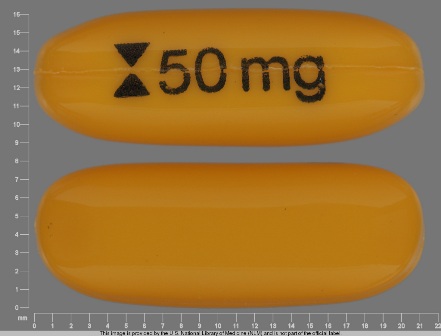
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0172-7311-46: 30 BLISTER PACK IN 1 CARTON (0172‑7311‑46) > 1 CAPSULE, LIQUID FILLED IN 1 BLISTER PACK (0172‑7311‑00)
Active Ingredients:
- Cyclosporine
Dosage Strength:
- 50 mg
Inactive Ingredients:
- Alcohol
- .alpha.-tocopherol, Dl-
- Fd&c Blue No. 2
- Aluminum Oxide
- Gelatin
- Glycine
- Glycerin
- Polyoxyl 40 Hydrogenated Castor Oil
- Polyglyceryl-3 Oleate
- Polyglyceryl-10 Oleate
- Propylene Glycol
- Shellac
- Sorbitol
- Titanium Dioxide
- Ferric Oxide Yellow
Pharmaceutical Classes:
- Calcineurin Inhibitor Immunosuppressant [EPC]
- Calcineurin Inhibitors [MoA]
- Cytochrome P450 3A4 Inhibitors [MoA]
- P-Glycoprotein Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 0172-7310 Cyclosporine, Modified 25 mg Oral Capsule by Ivax Pharmaceuticals, Inc.
- 0172-7312 Cyclosporine, Modified 100 mg Oral Capsule by Ivax Pharmaceuticals, Inc.
- 0172-7313 Cyclosporine 100 mg/ml Oral Solution by Ivax Pharmaceuticals, Inc.
- 0185-0932 Cyclosporine, Modified 25 mg Oral Capsule by Eon Labs, Inc.
- 0185-0933 Cyclosporine, Modified 100 mg Oral Capsule by Eon Labs, Inc.
- 0378-8760 Cyclosporine .5 mg/ml Ophthalmic Emulsion by Mylan Pharmaceuticals Inc.
- 0517-0866 Cyclosporine 50 mg/ml Intravenous Injection, Solution by American Regent, Inc.
- 0574-0866 Cyclosporine 50 mg/ml Intravenous Injection, Solution by Paddock Laboratories, Inc.
- 0591-2222 Cyclosporine, Modified 25 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-2223 Cyclosporine, Modified 100 mg Oral Capsule by Watson Laboratories, Inc.
- 0591-2224 Cyclosporine 100 mg/ml Oral Solution by Watson Laboratories, Inc.
- 10702-808 Cyclosporine .5 mg/ml Ophthalmic Emulsion by Kvk-tech, Inc.
- 23155-837 Cyclosporine 25 mg Oral Capsule, Liquid Filled by Avet Pharmaceuticals Inc
- 23155-838 Cyclosporine 50 mg Oral Capsule, Liquid Filled by Avet Pharmaceuticals Inc
- 23155-839 Cyclosporine 100 mg Oral Capsule, Liquid Filled by Avet Pharmaceuticals Inc
- 50090-5977 Cyclosporine .5 mg/ml Ophthalmic Emulsion by A-s Medication Solutions
- 51862-458 Cyclosporine 25 mg Oral Capsule, Liquid Filled by Mayne Pharma Inc.
- 51862-460 Cyclosporine 100 mg Oral Capsule, Liquid Filled by Mayne Pharma Inc.
- 54868-5522 Cyclosporine 100 mg Oral Capsule by Physicians Total Care, Inc.
- 54868-6232 Cyclosporine, Modified 100 mg Oral Capsule by Physicians Total Care, Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0172-7310Next: 0172-7312 >
Related Discussions:
cyclosporine capsule
My dog used to take Teva's 100 mg Cyclosporine. My pharmacy gave me a brand by Mayne pharma and now she is puking no...
My dog used to take Teva's 100 mg Cyclosporine. My pharmacy gave me a brand by Mayne pharma and now she is puking no...
cyclosporine 79mcgL is OK in human
I haved kidney transplant and my cyclosporine is a 79mc/L. is any concequense when is low of 100...
I haved kidney transplant and my cyclosporine is a 79mc/L. is any concequense when is low of 100...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.