0143-3367 : Flurazepam Hydrochloride 15 mg Oral Capsule
| NDC: | 0143-3367 |
| Labeler: | West-ward Pharmaceutical Corp |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Flurazepam Flurazepam |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA077107 |
| Rev. Date: | |
| CSA Schedule: | CIV (US) [1] |
[1] Schedule IV Controlled Substance: Low potential for abuse relative to substances in Schedule III. Examples include Alprazolam (Xanax), Diazepam (Valium), Carisoprodol (Soma), Clonazepam (Klonopin), Lorazepam (Ativan), Clorazepate (Tranxene), Midazolam (Versed), Temazepam (Restoril), and Triazolam (Halcion).. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | West;ward;Flurazepam;15 |
| Shapes: |
Capsule |
| Colors: |
 Blue Blue |
| Size (mm): | 18 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
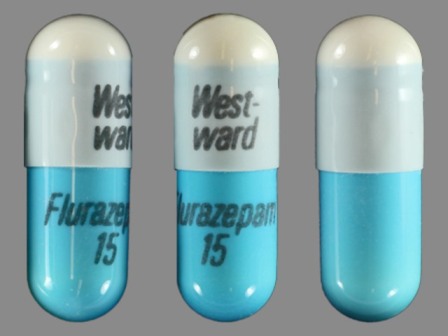
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0143-3367-01: 100 CAPSULE IN 1 BOTTLE, PLASTIC (0143‑3367‑01)
- 0143-3367-05: 500 CAPSULE IN 1 BOTTLE, PLASTIC (0143‑3367‑05)
- 0143-3367-30: 30 CAPSULE IN 1 BOTTLE, PLASTIC (0143‑3367‑30)
Active Ingredients:
- Flurazepam Hydrochloride
Dosage Strength:
- 15 mg
Inactive Ingredients:
- Lactose Monohydrate
- Magnesium Stearate
- Starch, Pregelatinized Corn
- Sodium Starch Glycolate Type a Potato
- Colloidal Silicon Dioxide
- Fd&c Blue No. 1
- Fd&c Red No. 3
- Gelatin
- Silicon Dioxide
- Sodium Lauryl Sulfate
- Titanium Dioxide
Pharmaceutical Classes:
- Benzodiazepine [EPC]
- Benzodiazepines [CS]
Related Products:
Based on records with the same trade name.- 0143-3370 Flurazepam Hydrochloride 30 mg Oral Capsule by West-ward Pharmaceutical Corp
- 21695-363 Flurazepam Hydrochloride 30 mg Oral Capsule by Rebel Distributors Corp
- 52959-236 Flurazepam Hydrochloride 30 mg Oral Capsule by H.j. Harkins Company, Inc.
- 54569-0898 Flurazepam Hydrochloride 30 mg Oral Capsule by A-s Medication Solutions LLC
- 54569-2376 Flurazepam Hydrochloride 15 mg Oral Capsule by A-s Medication Solutions LLC
- 60429-542 Flurazepam Hydrochloride 15 mg Oral Capsule by Golden State Medical Supply, Inc.
- 60429-543 Flurazepam Hydrochloride 30 mg Oral Capsule by Golden State Medical Supply, Inc.
- 63629-3034 Flurazepam Hydrochloride 15 mg Oral Capsule by Bryant Ranch Prepack
- 63629-3200 Flurazepam Hydrochloride 30 mg Oral Capsule by Bryant Ranch Prepack
- 68788-0408 Flurazepam Hydrochloride 30 mg Oral Capsule by Preferred Pharmaceuticals, Inc
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0143-3190Next: 0143-3370 >
Related Discussions:
flurazepam 30
blue casule ## I have a bottle of flurazepam 30 mg, I found in my drawer from 2010 when I suffered from insomnia back th... 3 replies
blue casule ## I have a bottle of flurazepam 30 mg, I found in my drawer from 2010 when I suffered from insomnia back th... 3 replies
Is Flurazepam still available?
I have been on Flurazepam for some time and am being told by my pharmacy that there is a manufacture problem and they do... 12 replies
I have been on Flurazepam for some time and am being told by my pharmacy that there is a manufacture problem and they do... 12 replies
Chartwell Flurazepam
Is anyone retaking Chartwell Flurazepam and getting the same results as they had with Mylan? It did not work for me. I&#...
Is anyone retaking Chartwell Flurazepam and getting the same results as they had with Mylan? It did not work for me. I&#...
Mylan Discontinued Flurazepam
Desperate times again. What to do? Where to go? This is absurd. Now back where we were 2 years ago, no one else makes it... 20 replies
Desperate times again. What to do? Where to go? This is absurd. Now back where we were 2 years ago, no one else makes it... 20 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.