0093-5551 : Dexmethylphenidate Hydrochloride 10 mg Oral Capsule, Extended Release
| NDC: | 0093-5551 |
| Labeler: | Teva Pharmaceuticals USA Inc |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Dexmethylphenidate Hydrochloride Dexmethylphenidate Hydrochloride |
| Dosage Form: | Oral Capsule, Extended Release |
| Application #: | ANDA078908 |
| Rev. Date: | |
| CSA Schedule: | CII (US) [1] |
[1] Schedule II / IIN Controlled Substance: High potential for abuse which may lead to severe psychological or physical dependence. (i.e. Narcotics such as Dilaudid, Methadone, Demerol, Oxycodone, Percocet, Fentanyl, Morphine, Opium, Codeine, and Hydrocodone ... Schedule IIN stimulants include non-narcotic Amphetamines such as Dexedrine, Adderall, Desoxyn, Methylphenidate (Ritalin) ... Other Schedule II substances include Amobarbital, Glutethimide, and Pentobarbital. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | TEVA;5551 |
| Shapes: |
Capsule |
| Colors: |
 White White |
| Size (mm): | 18 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
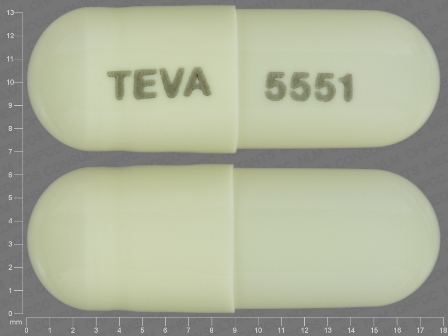
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0093-5551-01: 100 CAPSULE, EXTENDED RELEASE IN 1 BOTTLE (0093‑5551‑01)
Active Ingredients:
- Dexmethylphenidate Hydrochloride
Dosage Strength:
- 10 mg
Inactive Ingredients:
- Ammonio Methacrylate Copolymer Type B
- D&c Yellow No. 10
- Aluminum Oxide
- Fd&c Blue No. 1
- Fd&c Blue No. 2
- Indigotindisulfonate Sodium
- Fd&c Red No. 40
- Gelatin
- Hypromellose 2208 (3 Mpa.s)
- Ferrosoferric Oxide
- Methacrylic Acid - Methyl Methacrylate Copolymer (1:1)
- Starch, Corn
- Sucrose
- Polyethylene Glycol 6000
- Propylene Glycol
- Shellac
- Talc
- Titanium Dioxide
- Triethyl Citrate
Pharmaceutical Classes:
- Central Nervous System Stimulant [EPC]
- Central Nervous System Stimulation [PE]
Related Products:
Based on records with the same trade name.- 0093-5275 Dexmethylphenidate Hydrochloride 2.5 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5276 Dexmethylphenidate Hydrochloride 5 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5277 Dexmethylphenidate Hydrochloride 10 mg Oral Tablet by Teva Pharmaceuticals USA Inc
- 0093-5550 Dexmethylphenidate Hydrochloride 5 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5552 Dexmethylphenidate Hydrochloride 15 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5553 Dexmethylphenidate Hydrochloride 20 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5554 Dexmethylphenidate Hydrochloride 30 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5562 Dexmethylphenidate Hydrochloride 40 mg/1 Oral Capsule, Extended Release by Teva Pharmaceuticals USA Inc
- 0093-5045 Dexmethylphenidate Hydrochloride 25 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA, Inc.
- 0093-5046 Dexmethylphenidate Hydrochloride 35 mg Oral Capsule, Extended Release by Teva Pharmaceuticals USA, Inc.
- 0115-1682 Dexmethylphenidate Hydrochloride 5 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1683 Dexmethylphenidate Hydrochloride 10 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1684 Dexmethylphenidate Hydrochloride 15 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1685 Dexmethylphenidate Hydrochloride 20 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1686 Dexmethylphenidate Hydrochloride 30 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1709 Dexmethylphenidate Hydrochloride 25 mg Oral Capsule, Extended Release by Impax Generics
- 0115-1710 Dexmethylphenidate Hydrochloride 35 mg Oral Capsule, Extended Release by Impax Generics
- 0115-9918 Dexmethylphenidate Hydrochloride 5 mg Oral Capsule, Extended Release by Impax Generics
- 0115-9919 Dexmethylphenidate Hydrochloride 10 mg Oral Capsule, Extended Release by Impax Generics
- 0115-9920 Dexmethylphenidate Hydrochloride 15 mg Oral Capsule, Extended Release by Impax Generics
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0093-5550Next: 0093-5552 >
Related Discussions:
can you take dexmethylphenidate and gabapentin together
can you take 20mg of Dexmethylphenidate with 100 mg Gabapentin? I want to make sure that my son can take both at the sam... 1 reply
can you take 20mg of Dexmethylphenidate with 100 mg Gabapentin? I want to make sure that my son can take both at the sam... 1 reply
is dexmethylph the same as dexmethylphenidate hcl
is dexmethylph the same as dexmethylphenidate hcl? The pills in each bottle look alike both have a "D" on one si... 1 reply
is dexmethylph the same as dexmethylphenidate hcl? The pills in each bottle look alike both have a "D" on one si... 1 reply
Dexmethylphenidate & Guanfacine for adolescent
My 12 year old daughter is on both of these medications and from what I understand they are to treat the same thing. Is ...
My 12 year old daughter is on both of these medications and from what I understand they are to treat the same thing. Is ...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.