0093-5049 : Metformin Hydrochloride 500 mg / Pioglitazone 15 mg Oral Tablet
| NDC: | 0093-5049 |
| Labeler: | Teva Pharmaceuticals USA |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Pioglitazone and Metformin Hydrocholride Pioglitazone and Metformin Hydrocholride |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | NDA021842 |
| Rev. Date: |
Appearance:
| Markings: | 4833M;15;500 |
| Shapes: |
Oval |
| Colors: |
 White White |
| Size (mm): | 14 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
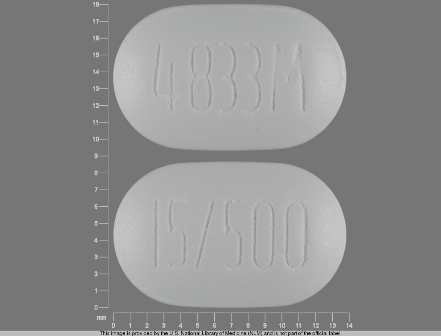
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0093-5049-06: 60 TABLET, FILM COATED IN 1 BOTTLE (0093‑5049‑06)
- 0093-5049-86: 180 TABLET, FILM COATED IN 1 BOTTLE (0093‑5049‑86)
Active Ingredients:
- Metformin Hydrochloride
- Pioglitazone Hydrochloride
Dosage Strength:
- 500 mg
- 15 mg
Inactive Ingredients:
- Povidones
- Cellulose, Microcrystalline
- Croscarmellose Sodium
- Magnesium Stearate
- Hypromellose 2910 (6 Mpa.s)
- Polyethylene Glycol 8000
- Titanium Dioxide
- Talc
Pharmaceutical Classes:
- Peroxisome Proliferator Receptor alpha Agonist [EPC]
- Peroxisome Proliferator Receptor gamma Agonist [EPC]
- Peroxisome Proliferator-activated Receptor Activity [MoA]
- PPAR alpha [Chemical/Ingredient]
- PPAR gamma [Chemical/Ingredient]
- Thiazolidinedione [EPC]
- Thiazolidinediones [Chemical/Ingredient]
- Biguanide [EPC]
- Biguanides [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0093-5050 Metformin Hydrochloride 850 mg / Pioglitazone 15 mg Oral Tablet by Teva Pharmaceuticals USA
- 42291-693 Pioglitazone and Metformin Hydrocholride Oral Tablet, Film Coated by Avkare, Inc.
- 42291-694 Pioglitazone and Metformin Hydrocholride Oral Tablet, Film Coated by Avkare, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0093-5046Next: 0093-5050 >
Related Discussions:
what are the side effects of pioglitazone hydrochloride plus metaphormin
I AM A SUGAR PATIENT AND DR PRESCRIBED ME PIOZMF-30 ABOUT 2 YEARS BACK. IMMEDIATELLY AFTER A MONTH OF USE I DEVELOPED SK... 1 reply
I AM A SUGAR PATIENT AND DR PRESCRIBED ME PIOZMF-30 ABOUT 2 YEARS BACK. IMMEDIATELLY AFTER A MONTH OF USE I DEVELOPED SK... 1 reply
Metformin Hydrochloride and Sitagliptin Phosphate made in India
Please tell me the name of an Indian medicine similar to Metformin Hydrochloride + Sitagliptin Phosphate. For a low cost... 3 replies
Please tell me the name of an Indian medicine similar to Metformin Hydrochloride + Sitagliptin Phosphate. For a low cost... 3 replies
METFORMIN HYDROCHLORIDE
DIABETES MEDICINE ## IAM TAKING THIS TABLETS 3 TIMES DAILY AND WANT TO KNOW HOW AND WHAT WAY THIS TABLET WILL HELP ME? t... 2 replies
DIABETES MEDICINE ## IAM TAKING THIS TABLETS 3 TIMES DAILY AND WANT TO KNOW HOW AND WHAT WAY THIS TABLET WILL HELP ME? t... 2 replies
Metformin Hydrochloride i p - 1000mg + Methylcobalamin 750 mcg
Please let us know under what brand names the above-noted composition is available in the markets of Delhi & the nam... 2 replies
Please let us know under what brand names the above-noted composition is available in the markets of Delhi & the nam... 2 replies
metformin hydrochloride prolonged release tablets up 500 mg
Metformin hydrochloride prolonged release tablets Ip 500 mg Melmet_500Sr for what disease? ## Hello, Rani! How are you? ... 1 reply
Metformin hydrochloride prolonged release tablets Ip 500 mg Melmet_500Sr for what disease? ## Hello, Rani! How are you? ... 1 reply
Metformin Hydrochloride + Sitagliptin Phosphate side effects?
What are the effects of these medications? ## Both Metformin and Sitagliptin are oral antidiabetic medications, used to ... 1 reply
What are the effects of these medications? ## Both Metformin and Sitagliptin are oral antidiabetic medications, used to ... 1 reply
Co-Metformin & Metformin Hydrochloride.
I have a prescription which reads (360 TAB) Co-Metformin 500mg; Metformin Hydrochloride 500mg. Does this mean that there... 2 replies
I have a prescription which reads (360 TAB) Co-Metformin 500mg; Metformin Hydrochloride 500mg. Does this mean that there... 2 replies
Gliclazide Metformin Hydrochloride Tablet In India
Can I use GLIZET(500 mg-Gliclazide Metformin Hydrochloride Tablet) instead of glycinorm-M80 BD? ## From what I'm fin... 1 reply
Can I use GLIZET(500 mg-Gliclazide Metformin Hydrochloride Tablet) instead of glycinorm-M80 BD? ## From what I'm fin... 1 reply
Gliclazide Metformin Hydrochloride Tablet
Pl let me know the doses for Gliclazide Metformin Hydrochloride tablet ## What country are you in? This formulation isn&... 1 reply
Pl let me know the doses for Gliclazide Metformin Hydrochloride tablet ## What country are you in? This formulation isn&... 1 reply
Glimepiride Metformin Hydrochloride Extended Release Tablets
This medicine have any side effects because it is a long time use medicine ## All medications carry the risk of side eff... 3 replies
This medicine have any side effects because it is a long time use medicine ## All medications carry the risk of side eff... 3 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.