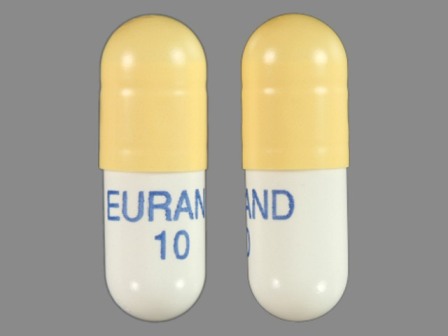
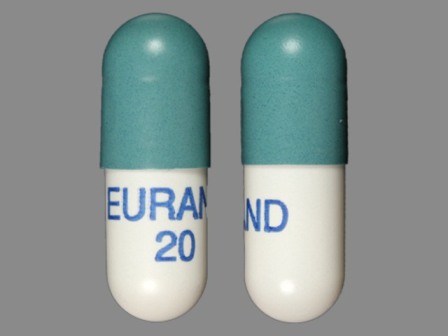
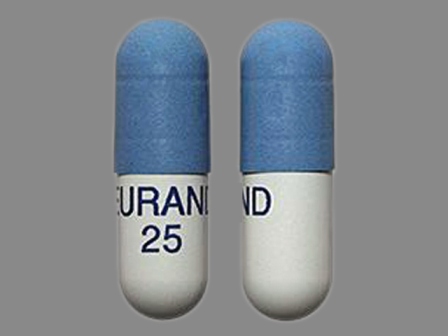
Zenpep
Active Ingredient(s): PancrelipaseFDA Approved: * August 27, 2009
Pharm Company: * EURAND
Category: Enzymes
Pancreatic enzymes, also known as pancreases or pancrelipase and pancreatin, are commercial mixtures of amylase, lipase, and protease.[1][2] They are used to treat malabsorption syndrome due to certain pancreatic problems.[1] These pancreatic problems may be due to cystic fibrosis, surgical removal of the pancreas, long term pancreatitis, or pancreatic cancer, among others.[1][3] The preparation is taken by mouth.... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.6 Discussions
Dosage List
Related Brands
Drugs with the same active ingredientsPopular Topics
Has anyone been approved for Actavis Patient Assistance Program? I've been in the coverage gap (donut hole) since Fe...
Yes, all non-approved pancrelipase drug products have stopped being manufactured and being sold to wholesalers and pharm...
Last week the P/A increased my dosage of Zenpep from 5,000 units a meal to 50,000 units a meal. It has not helped. I wil...
Activas Patient Assistance Program does not offer any assistance while in the coverage gap. Creon manufacturer does offe...