Videx EC
Active Ingredient(s): Didanosine, ddIFDA Approved: * October 31, 2000
Pharm Company: * BRISTOL MYERS SQUIBB
Category: HIV / AIDS
Didanosine (ddI, DDI), sold under the brand name Videx, is a medication used to treat HIV/AIDS.[1] It is used in combination with other medications as part of highly active antiretroviral therapy (HAART). It is of the reverse-transcriptase inhibitor class. Didanosine was first described in 1975 and approved for use in the United States in 1991.[2] Contents 1 Adverse effects 2 Drug interactions 3 Resistance 4 Mechanism of action 5 Pharmacokinetics 6 History 7 ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
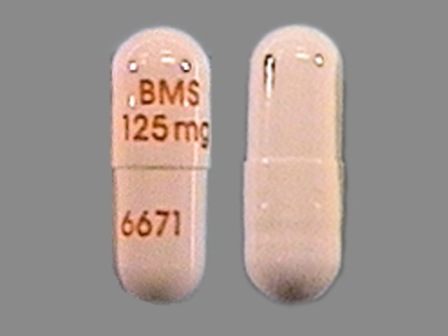
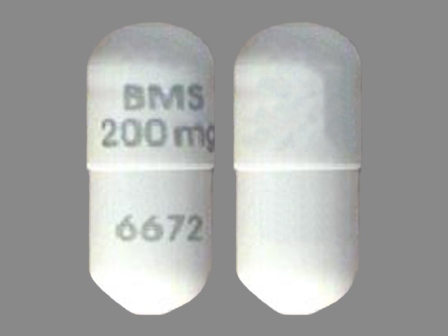
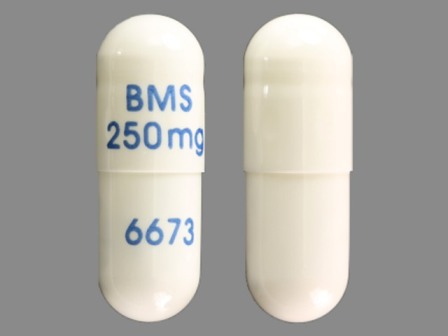
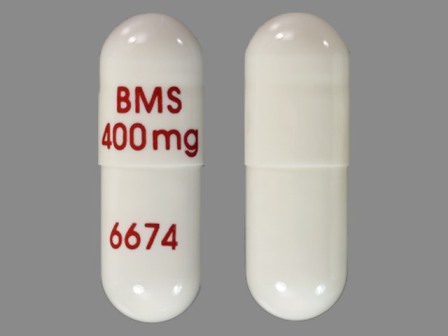
Related Brands
Drugs with the same active ingredientsPopular Topics
They are like a gold color. the meduim sizes. ## WHAT ARE THE SIDE EFFECTS AND DOES HELP YOU GAIN WEIGHT ## WHAT ARE THE...
Hi. Please let me know what Ecospirin 75 is indicated for? My Doc has advised Ecospirin 75 to my father -age (65)- after...
I'm in my 21st week of pregnancy and my doc advised me to take ecosprin 75 before sleeping. She said I had a little ...
I am in the 8th month of my pregnancy. Last week when we went for regular check up, I was give Ecosprin 75 and Duvadilan...
What are the side effects of ecosprin AV 150? Can it cause nose bleeds? ## Ecosprin contains Aspirin and Atorvastatin, i...
i am taking ecosprin gold 10 for 5 years. Please let me know its side effects. ## Sir,CABGS was done on 10-09-2009 . I u...
What is this medicine given for? ## Econorm contains Saccharomyces Boulardil, it is used to treat IBS. Do you have any o...
what is the effect of ecosprin gold 20 on blood pressure ## Ecosprin Gold contain 75mgs of Aspirin, 10mgs of Atorvastati...
I have completed 28 weeks of my pregnancy. I had been advised to take Ecosprin 75 tablet daily after lunch. Is it safe? ...
Diabetis Mellitus type-II, osteoarthritis, on antidiabetic drug-glimipride- metformin composition and Sammy-200mg for os...