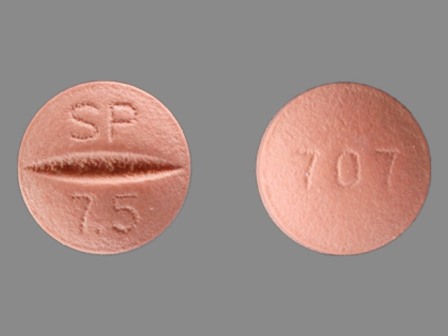
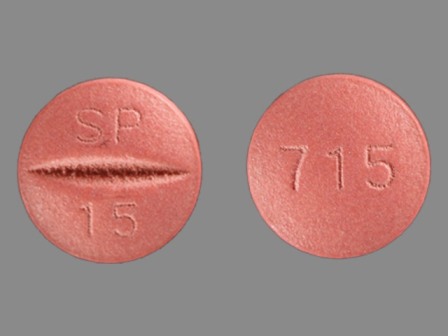
Univasc
Active Ingredient(s): MoexiprilFDA Approved: * April 19, 1995
Pharm Company: * SCHWARZ PHARMA
Category: Blood Pressure
Moexipril an angiotensin converting enzyme inhibitor (ACE inhibitor)[1] used for the treatment of hypertension and congestive heart failure. Moexipril can be administered alone or with other antihypertensives or diuretics.[2] It works by inhibiting the conversion of angiotensin I to angiotensin II.[3] It was patented in 1980 and approved for medical use in 1995.[4] Moexipril is available from Schwarz Pharma under the trade name... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
Popular Topics
Generic for Univasc
I once took Univasc in a generic brand, then it was discontinued. My Pharmacy informed me that they could no longer get ...