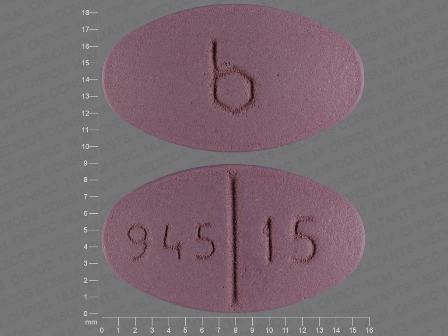
Trexall
Active Ingredient(s): MethotrexateFDA Approved: * March 21, 2001
Pharm Company: * BARR
Category: Cancer
Methotrexate (MTX), formerly known as amethopterin, is a chemotherapy agent and immune-system suppressant.[4] It is used to treat cancer, autoimmune diseases, and ectopic pregnancy and for medical abortions.[4] Types of cancers it is used for include breast cancer, leukemia, lung cancer, lymphoma, gestational trophoblastic disease, and osteosarcoma.[4] Types of autoimmune diseases it is used for include psoriasis, rheumatoid arthritis, and Crohn... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List