Tekamlo
Active Ingredient(s): Aliskiren + AmlodipineFDA Approved: * August 26, 2010
Pharm Company: * NOVARTIS PHARMS
Category: Blood Pressure
The drug combination aliskiren/amlodipine (INNs, trade names Tekamlo and Rasilamlo) is an antihypertensive. Clinical trials have shown it to be more effective than amlodipine on its own,[1] with a high dosing regime (aliskiren 300mg/amlodipine 10mg) being more effective than olmesartan/amlodipine with comparable tolerability.[2] References .mw-parser-output .reflist{font-size:90%;margin-bottom:0.5em;list-style-type:decimal}.mw-parser-output .reflist .referen... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List

Tekamlo 150/5 (Aliskiren / Amlodipine) Oral Tablet
NDC: 0078-0603
Labeler:
Novartis Pharmaceuticals Corporation
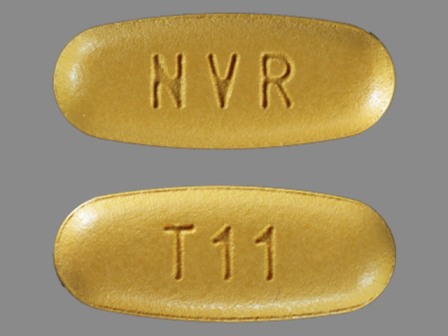
Tekamlo 300/5 (Aliskiren / Amlodipine) Oral Tablet
NDC: 0078-0605
Labeler:
Novartis Pharmaceuticals Corporation
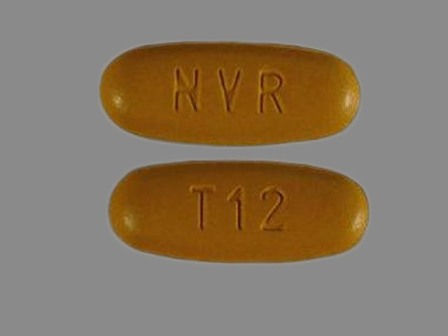
Tekamlo 300/10 (Aliskiren / Amlodipine) Oral Tablet
NDC: 0078-0606
Labeler:
Novartis Pharmaceuticals Corporation
Tekamlo 150/10 (Aliskiren / Amlodipine) Oral Tablet
NDC: 0078-0604
Labeler:
Novartis Pharmaceuticals Corporation