Targretin
Active Ingredient(s): BexaroteneFDA Approved: * December 29, 1999
Pharm Company: * LIGAND
Category: Cancer
Bexarotene, sold under the brand Targretin, is an antineoplastic (anti-cancer) agent approved by the U.S. Food and Drug Administration (FDA) (December 1999) and the European Medicines Agency (EMA) (March 2001) for use as a treatment for cutaneous T cell lymphoma (CTCL).[1] It is a third-generation retinoid.[2] Contents 1 Medical uses 2 Contraindications 3 Adverse effects 4 Interactions 5 Mechanism 6 Physical properties 7 History 8 References Medical uses... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
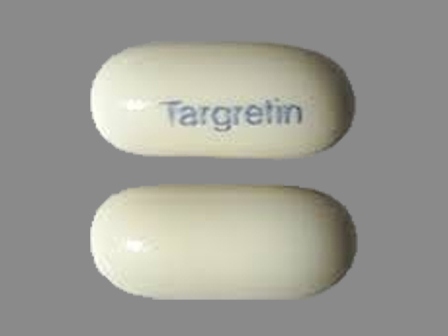
Targretin 75 mg/1 Oral Capsule, Liquid Filled
NDC: 0187-5526
Labeler:
Valeant Pharmaceuticals North America LLC
Popular Topics
Targretin for Cutaneous T Cell Lymphoma 2 REPLIES
2 REPLIES
My husband has CTCL and is being told to take Targretin. Can anyone tell me if they have taken it and what side effects ...