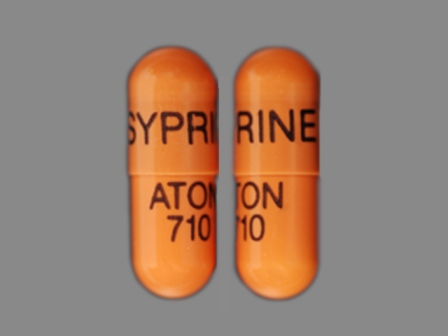
Syprine
Active Ingredient(s): TrientineFDA Approved: * November 8, 1985
Pharm Company: * MERCK
Category: Genetic Disorders
Triethylenetetramine (TETA and trien), also called trientine (INN), is an organic compound with the formula [CH2NHCH2CH2NH2]2. This oily liquid is colorless but, like many amines, older samples assume a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA.[9] Contents 1 Uses 1.1 Medical uses 2 Society and cul... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.