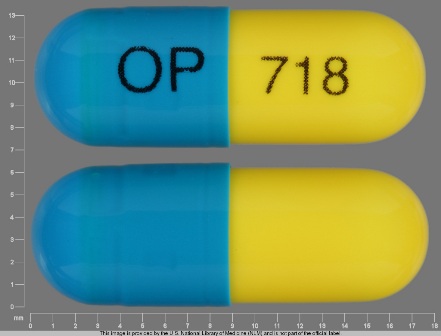
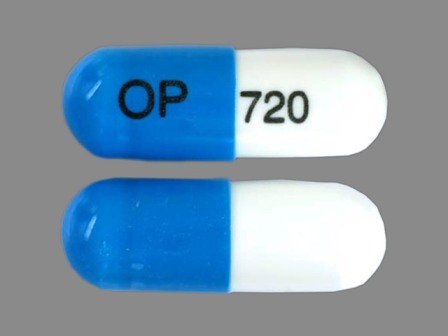
Surmontil
Active Ingredient(s): TrimipramineFDA Approved: * June 12, 1979
Pharm Company: * ODYSSEY PHARMS
Category: Antidepressant
Trimipramine, sold under the brand name Surmontil among others, is a tricyclic antidepressant (TCA) which is used to treat depression.[5][6][7][8] It has also been used for its sedative, anxiolytic, and weak antipsychotic effects in the treatment of insomnia, anxiety disorders, and psychosis, respectively.[5][6][7][8] The drug is described as an atypical or "second-g... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.