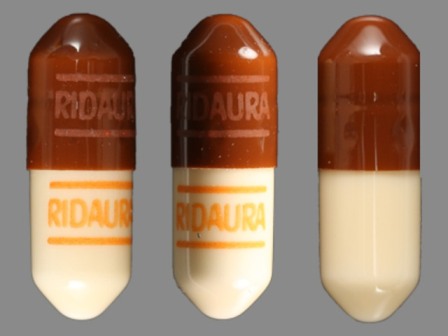
Ridaura
Active Ingredient(s): AuranofinFDA Approved: * May 24, 1985
Pharm Company: * PROMETHEUS LABS
Category: Arthritis
Auranofin is a gold salt classified by the World Health Organization as an antirheumatic agent. It has the brand name Ridaura. Contents 1 Use 2 Research 2.1 HIV infection 2.2 Amebiasis 2.3 Acanthamoeba Keratitis and Primary Amoebic Meningoencephalitis 2.4 Tuberculosis 2.5 Ovarian cancer 2.6 COVID-19 3 References 4 Further reading 5 External links Use Auranofin is used to treat rheumatoid arthritis. It improves arthritis symptoms including painful or tender and swollen joints and mo... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.