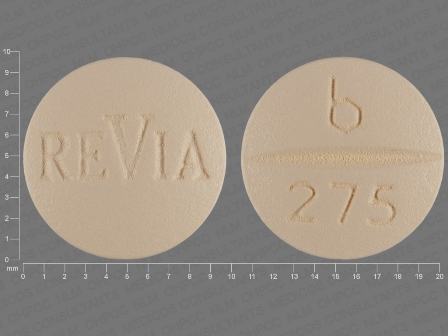
ReVia
Active Ingredient(s): NaltrexoneFDA Approved: * November 20, 1984
Pharm Company: * DURAMED
Category: Alcoholism
Naltrexone, sold under the brand names ReVia and Vivitrol among others, is a medication primarily used to manage alcohol or opioid use disorder by reducing cravings and feelings of euphoria associated with substance use disorder.[4] It has also been found to be effective in the treatment of other addictions and may be used for them off-label.[11] An opioid-dependent person should not receive naltrexone before detoxification.[4] It is taken by mo... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.4 Discussions
Dosage List