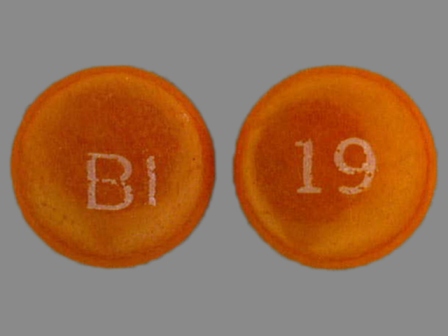
Persantine
Active Ingredient(s): DipyridamoleFDA Approved: * December 6, 1961
Pharm Company: * BOEHRINGER INGELHEIM
Category: Blood Thinner (Anticoagulant)
Dipyridamole (trademarked as Persantine and others) is a nucleoside transport inhibitor and a PDE3 inhibitor medication that inhibits blood clot formation[3] when given chronically and causes blood vessel dilation when given at high doses over a short time. Contents 1 Medical uses 1.1 Stroke 1.2 Other uses 2 Drug interactions 3 Overdose 4 Mechanisms of action 5 Experimental studies 6 See also 7 References Medical uses Dipyridamole is used to dilate blood vessels in p... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
Popular Topics
persantine for men
persantine...