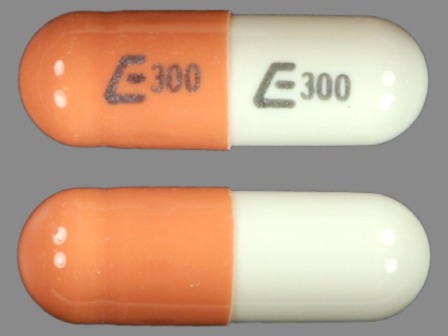
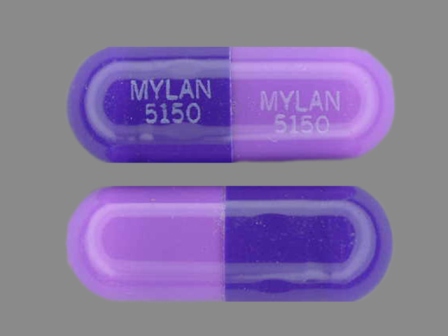
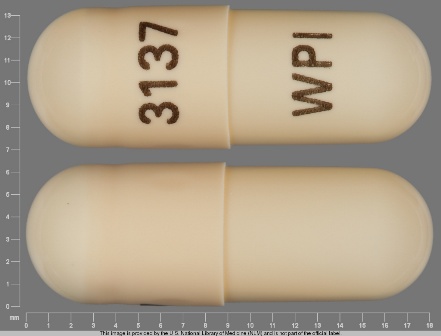
Nizatidine
FDA Approved: * July 5, 2002Pharm Company: * MYLAN
Category: Ulcer
Nizatidine is a histamine H2 receptor antagonist that inhibits stomach acid production, and is commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease.[1] It was patented in 1980 and approved for medical use in 1988.[2][3] It was developed by Eli Lilly. Brand names include Tazac and Axid. Contents 1 Medical use 2 Adverse effects 3 History and development 4 See also 5 References 6 External links Medical u... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.3 Discussions
Dosage List