Nisoldipine
FDA Approved: * July 25, 2008Pharm Company: * MYLAN
Category: Blood Pressure
Nisoldipine is a pharmaceutical drug used for the treatment of chronic angina pectoris and hypertension. It is a calcium channel blocker of the dihydropyridine class. It is sold in the United States under the proprietary name Sular. Nisoldipine has tropism for cardiac blood vessels.[1] It was patented in 1975 and approved for medical use in 1990.[2] Contents 1 Contraindications 2 Adverse effects 3 Interactions 4 Pharmacology 4.1 Mechanism of action 5 Refer... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
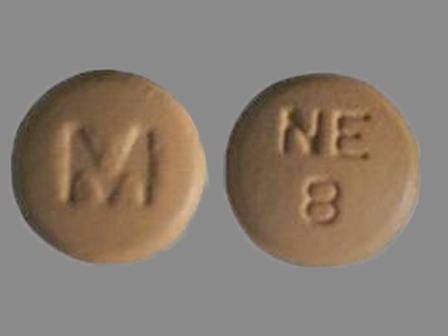
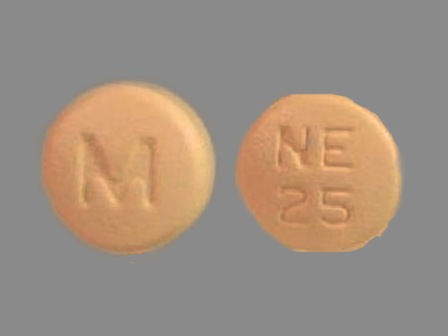
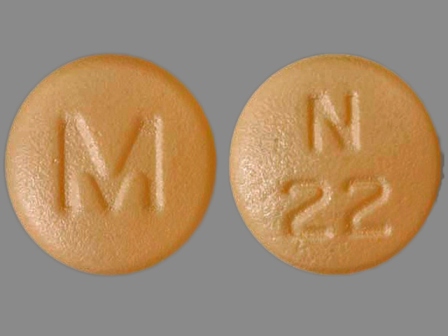
Nisoldipine 25.5 mg 24 Hr Extended Release Tablet
NDC: 0378-2098
Labeler:
Mylan Pharmaceuticals Inc.



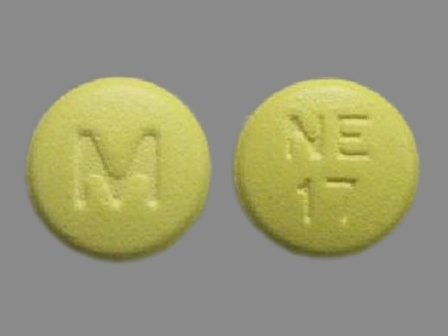
Nisoldipine 17 mg 24 Hr Extended Release Tablet
NDC: 0677-1979
Labeler:
United Research Laboratories, Inc.

Nisoldipine 25.5 mg 24 Hr Extended Release Tablet
NDC: 0677-1980
Labeler:
United Research Laboratories, Inc.

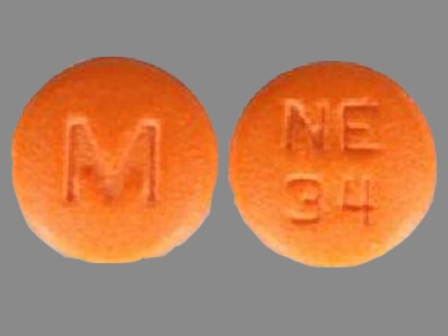
Nisoldipine 34 mg 24 Hr Extended Release Tablet
NDC: 0677-1981
Labeler:
United Research Laboratories, Inc.
Related Brands
Drugs with the same active ingredientsPopular Topics
Nisoldipine 8 mg 1 REPLY
1 REPLY
I was taking Losartan for a few months and my hair started to thin. Found out this is a side effect of Losartan. Asked d...
Nisoldipine
is there a generic for nisoldipine?...
Sular Nisoldipine Information 1 REPLY
1 REPLY
What can you tell me about the 20 mg tablet of Sular Nisoldipine? ## Sular Info Click Here...