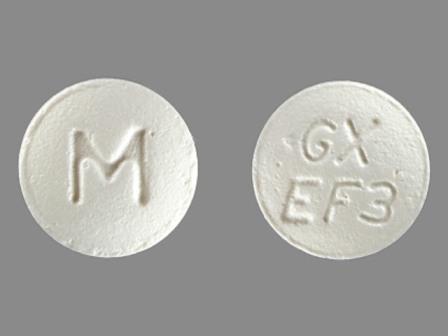
Myleran
Active Ingredient(s): BusulfanFDA Approved: * June 26, 1954
Pharm Company: * GLAXOSMITHKLINE
Category: Cancer
Busulfan (Myleran, GlaxoSmithKline, Busulfex IV, Otsuka America Pharmaceutical, Inc.) is a chemotherapy drug in use since 1959. It is a cell cycle non-specific alkylating antineoplastic agent, in the class of alkyl sulfonates. Its chemical designation is 1,4-butanediol dimethanesulfonate. Contents 1 History 2 Indications 3 Availability 4 Side effects 5 Dosing, administration, and pharmacokinetics 6 Drug interactions 7 Pharmacology 8 Complexation 9 References 10 External links History B... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List