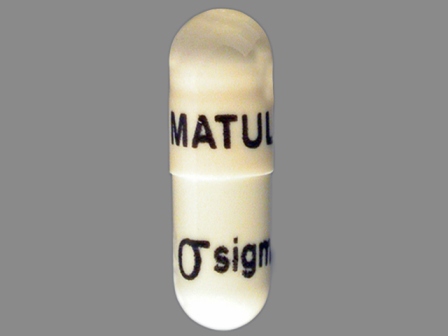
Matulane
Active Ingredient(s): ProcarbazineFDA Approved: * July 22, 1969
Pharm Company: * SIGMA TAU
Category: Cancer
Procarbazine is a chemotherapy medication used for the treatment of Hodgkin's lymphoma and brain cancers.[1] For Hodgkin's it is often used together with chlormethine, vincristine, and prednisone while for brain cancers such as glioblastoma multiforme it is used with lomustine and vincristine.[1] It is typically taken by mouth.[1] Common side effect include low blood cell counts and vomiting.[1] Other side effects include tiredne... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.