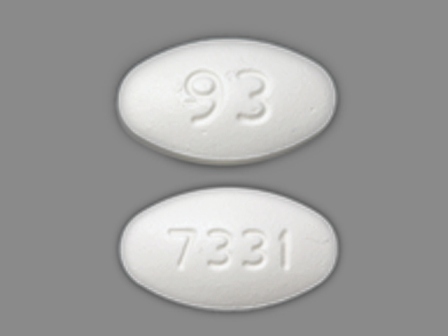
Lofibra
Active Ingredient(s): FenofibrateCategory: Cholesterol
Fenofibrate (sold under the brand names Tricor, Fenobrat etc.), is an oral medication of the fibrate class used to treat abnormal blood lipid levels.[2] It is less preferred to statin medications as it does not appear to reduce the risk of heart disease or death.[2][3] Its use is recommended together with dietary changes.[2] Common side effects include liver problems, breathing problems, abdominal pain, muscle problems, and nause... [wikipedia]
Dosage List