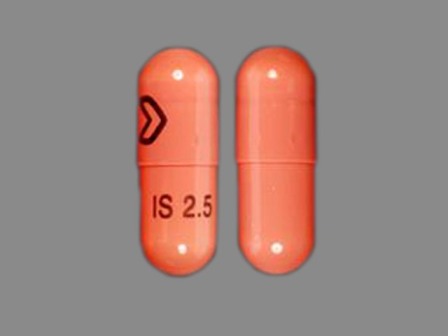
Isradipine
FDA Approved: * January 5, 2006Pharm Company: * ABRIKA PHARMS
Category: Blood Pressure
Isradipine (tradenames DynaCirc, Prescal) is a calcium channel blocker of the dihydropyridine class. It is usually prescribed for the treatment of high blood pressure in order to reduce the risk of stroke and heart attack. It was patented in 1978 and approved for medical use in 1989.[1] Contents 1 Medical uses 2 Side effects 3 Drug interactions 4 Overdose 5 Stereochemistry 6 References 7 Further reading 8 External links Medical uses Isradipine is given as either a 2.5mg... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List