Granisetron
FDA Approved: * December 31, 2007Pharm Company: * SICOR PHARMS
Category: Cancer
Granisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy and radiotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors. Granisetron was developed by chemists working at the British dr... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List

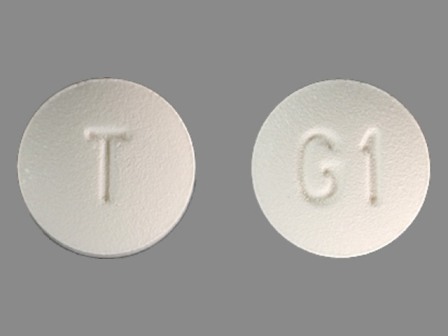
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 0093-7485
Labeler:
Teva Pharmaceuticals USA Inc
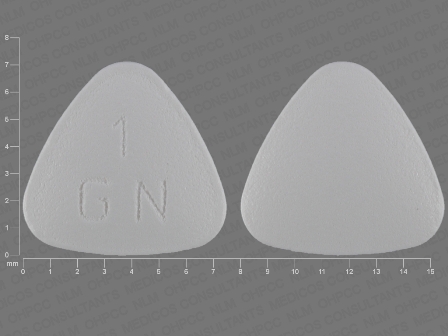
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 51672-4138
Labeler:
Taro Pharmaceuticals U.S.a., Inc.
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 51991-735
Labeler:
Breckenridge Pharmaceutical, Inc.

Granisetron Hydrochloride 1 mg Oral Tablet, Film Coated
NDC: 63850-0005
Labeler:
Natco Pharma Limited

Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 67877-184
Labeler:
Ascend Laboratories, LLC

Granisetron Hydrochloride 1 mg Oral Tablet, Film Coated
NDC: 69189-4138
Labeler:
Avera Mckennan Hospital
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 0054-0143
Labeler:
Roxane Laboratories, Inc
Granisetron Hydrochloride 1 mg/ml Intravenous Solution
NDC: 0143-9744
Labeler:
West-ward Pharmaceutical Corp
Granisetron Hydrochloride 4 mg/4ml Intravenous Solution
NDC: 0143-9745
Labeler:
West-ward Pharmaceutical Corp
Granisetron 1 mg (Granisetron Hydrochloride 1.12 mg) Oral Tablet
NDC: 16714-221
Labeler:
Northstar Rx LLC