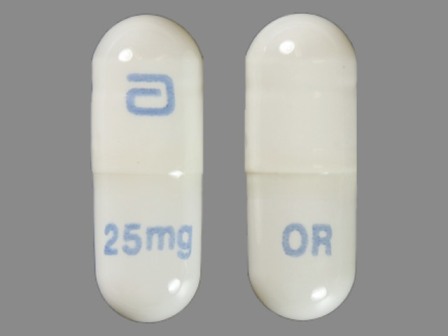
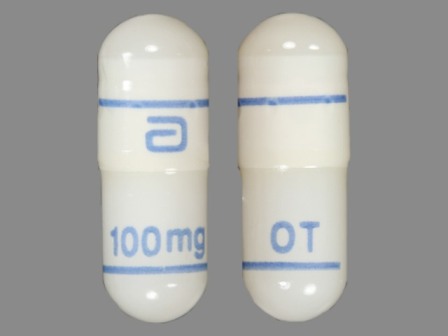
Gengraf
Active Ingredient(s): CyclosporineFDA Approved: * May 12, 2000
Pharm Company: * ABBOTT
Category: Immunosuppressive
Ciclosporin, also spelled cyclosporine and cyclosporin, is a calcineurin inhibitor, used as an immunosuppressant medication. It is a natural product.[6] It is taken by mouth or by injection into a vein for rheumatoid arthritis, psoriasis, Crohn's disease, nephrotic syndrome, and in organ transplants to prevent rejection.[6][7] It is also used as eye drops for keratoconjunctivitis sicca (dry eyes).[8] Common side effects include h... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List