Estropipate
FDA Approved: * September 23, 1993Pharm Company: * WATSON LABS
Category: Women's Health / Fertility
Estropipate, also known as piperazine estrone sulfate and sold under the brand names Harmogen, Improvera, Ogen, Ortho-Est, and Sulestrex among others, is an estrogen medication which is used mainly in menopausal hormone therapy in the treatment of menopausal symptoms.[1][2][3][4] It is a salt of estrone sulfate and piperazine, and is transformed into estrone and estradiol in the body.[2][3] It is t... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.3 Discussions
Dosage List
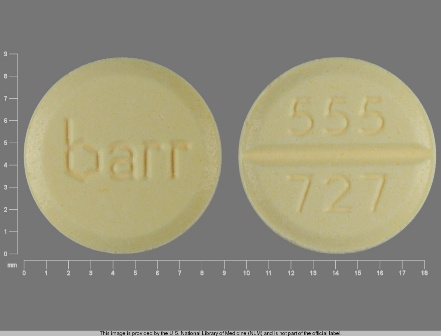
Estropipate 0.75 mg (Sodium Estrone Sulfate 0.625 mg) Oral Tablet
NDC: 0555-0727
Labeler:
Barr Laboratories Inc.

Estropipate 0.75 mg (Sodium Estrone Sulfate 0.625 mg) Oral Tablet
NDC: 0591-0414
Labeler:
Watson Laboratories, Inc.

Estropipate 1.5 mg (Sodium Estrone Sulfate 1.25 mg) Oral Tablet
NDC: 0591-0415
Labeler:
Watson Laboratories, Inc.

Estropipate 3 mg (Sodium Estrone Sulfate 2.5 mg) Oral Tablet
NDC: 0591-0416
Labeler:
Watson Laboratories, Inc.


Estropipate 1.5 mg (Sodium Estrone Sulfate 1.25 mg) Oral Tablet
NDC: 0555-0728
Labeler:
Barr Laboratories Inc.
Estropipate 3 mg (Sodium Estrone Sulfate 2.5 mg) Oral Tablet
NDC: 0555-0729
Labeler:
Barr Laboratories Inc.
Estropipate 0.75 mg (Sodium Estrone Sulfate 0.625 mg) Oral Tablet
NDC: 54868-3114
Labeler:
Physicians Total Care, Inc.
Estropipate 1.5 mg (Sodium Estrone Sulfate 1.25 mg) Oral Tablet
NDC: 54868-4149
Labeler:
Physicians Total Care, Inc.
Popular Topics
Estropipate Availability 2019 6 REPLIES
6 REPLIES
Did they stop making Estropipate? If not, can anyone please tell me about its current availability? I love this drug. Be...