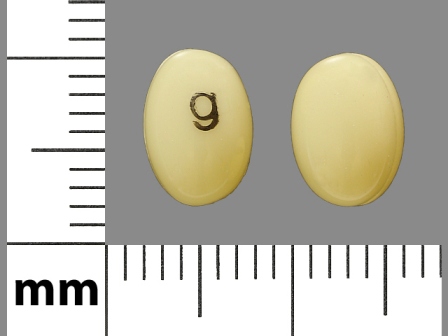
Doxercalciferol
FDA Approved: * August 30, 2013Pharm Company: * MYLAN PHARMA
Category: Thyroid
Doxercalciferol (or 1-hydroxyergocalciferol, trade name Hectorol) is drug for secondary hyperparathyroidism and metabolic bone disease.[1] It is a synthetic analog of ergocalciferol (vitamin D2). It suppresses parathyroid synthesis and secretion.[2] Docercalciferol is the vitamin D2 analogue of alfacalcidol.[3] It undergoes 25-hydroxylation in the liver to become the active ercalcitriol, without the involvement of kidneys.[4] ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List