Dipentum
Active Ingredient(s): OlsalazineFDA Approved: * July 31, 1990
Pharm Company: * UCB INC
Category: Anti-Inflammatory
Olsalazine is an anti-inflammatory medication used in the treatment of ulcerative colitis.[2][3] It is sold under the brand name Dipentum.[4] Olsalazine itself is a pro-drug of mesalazine (5-aminosalicyclic acid or 5-ASA) and is not absorbed in the small intestine. Instead it continues through to the colon where it is cleaved into two molecules of 5-ASA by azoreductases produced by colonic bacteria. Olsalazine thus exerts its anti-inflammatory e... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.2 Discussions
Dosage List
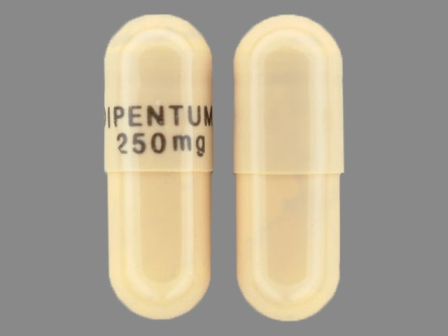
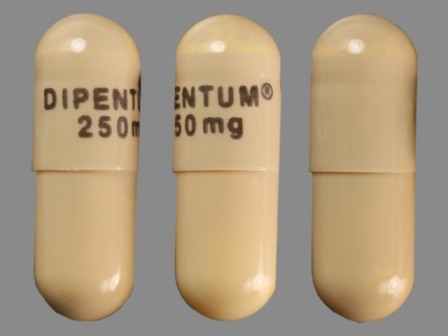

Popular Topics
dipentum, cost 2 REPLIES
2 REPLIES
Each year for the last several my co-pay for dipentum has increased dramatically, now to $250 per month from about $40. ...
dipentum verses sufasalazine
lost job can no longer aford high cost of dipentum for my chrons disease am wondering if sufasalazine will do as well as...