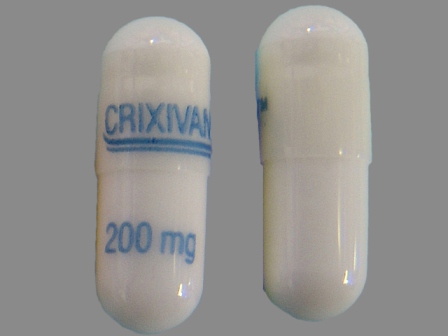
Crixivan
Active Ingredient(s): IndinavirFDA Approved: * March 13, 1996
Pharm Company: * MERCK
Category: HIV / AIDS
Indinavir (IDV; trade name Crixivan, made by Merck) is a protease inhibitor used as a component of highly active antiretroviral therapy to treat HIV/AIDS. It is soluble white powder administered orally in combination with other antiviral drugs. The drug prevents protease from functioning normally. Consequently, HIV viruses cannot reproduce, causing a decrease in the viral load. Commercially sold indinavir is indinavir anhydrous, which is indinavir with an additional amine in the hydroxyethyle... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List