Combivir
Active Ingredient(s): Lamivudine, 3TC + Zidovudine, ZDVFDA Approved: * September 26, 1997
Pharm Company: * GLAXOSMITHKLINE
Category: HIV / AIDS
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS.[5] It is generally recommended for use in combination with other antiretrovirals.[5] It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure.[5] It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine.[5]... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.4 Discussions
Dosage List
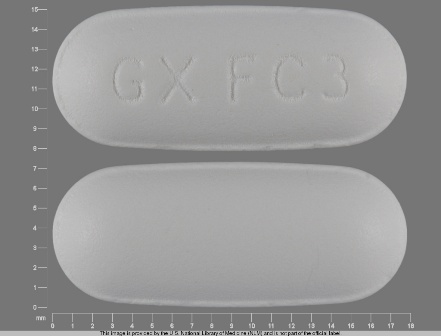
Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 0173-0595
Labeler:
Glaxosmithkline LLC

Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 49702-202
Labeler:
Viiv Healthcare Company

Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 11819-359
Labeler:
Hhs/Program Support Center/Supply Service Center


Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 21695-846
Labeler:
Rebel Distributors Corp


Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 49349-683
Labeler:
Remedyrepack Inc.


Combivir (Lamivudine 150 mg / Zidovudine 300 mg) Oral Tablet
NDC: 52959-546
Labeler:
H.j. Harkins Company, Inc.

Popular Topics
Cannot get Combivir
I am currently working in SE Asia and may have been exposed to HIV. I cannot get combivir but did find in pill form the ...