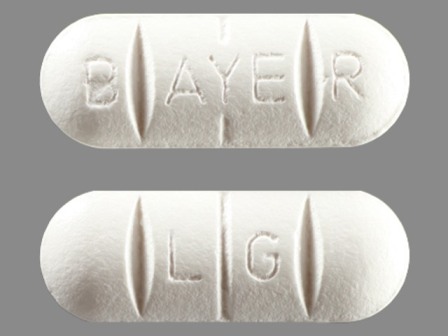
Biltricide
Active Ingredient(s): PraziquantelFDA Approved: * December 29, 1982
Pharm Company: * BAYER PHARMS
Category: Antiprotozoal / Anthelmintic
Praziquantel (PZQ), sold under the brandname Biltricide among others, is a medication used to treat a number of types of parasitic worm infections in mammals, birds, amphibians, reptiles, and fish.[2] In humans specifically, it is used to treat schistosomiasis, clonorchiasis, opisthorchiasis, tapeworm infections, cysticercosis, hydatid disease, and other fluke infections.[2] It should not be used for worm infections of the eye.[3] It is taken by... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.