Apriso
Active Ingredient(s): MesalamineFDA Approved: * October 31, 2008
Pharm Company: * SALIX PHARMS
Category: Anti-Inflammatory
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a medication used to treat inflammatory bowel disease, including ulcerative colitis and Crohn's disease.[1] It is generally used for mildly to moderately severe disease.[1] It is taken by mouth or rectally.[1] The formulations which are taken by mouth appear to be similarly effective.[4] Common side effects include headache, nausea, abdominal pain, and feve... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
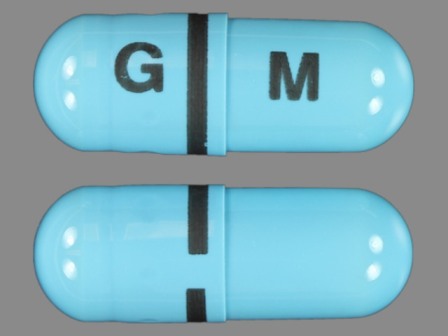

Apriso 375 mg Oral Capsule, Extended Release
NDC: 43353-884
Labeler:
Aphena Pharma Solutions - Tennessee, LLC
