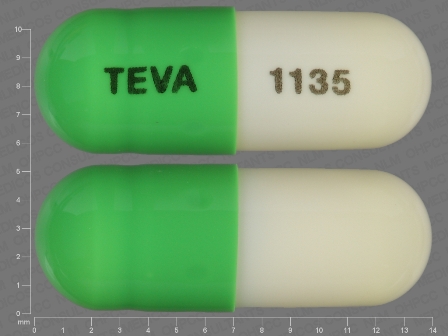
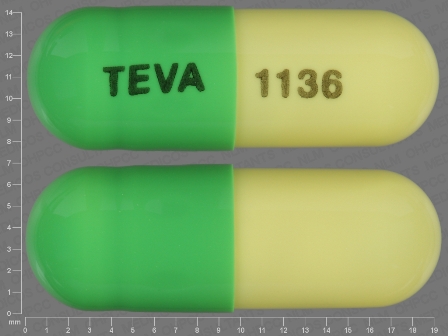
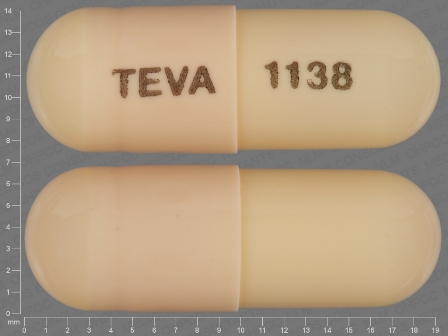
Acitretin
FDA Approved: * April 4, 2013Pharm Company: * BARR LABS INC
Category: Skin Care
Acitretin (trade names Soriatane and Neotigason) is a second-generation retinoid. It is taken orally, and is typically used for psoriasis.[1] Acitretin is an oral retinoid used in the treatment of severe resistant psoriasis. Because of the potential for problems and severe side effects it is generally used in only very severe cases of psoriasis that have been unresponsive to other treatments. It binds to nuclear receptors that regulates gene transcription. They induce kerat... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List