Cti 102 Pill
35 Topics Found
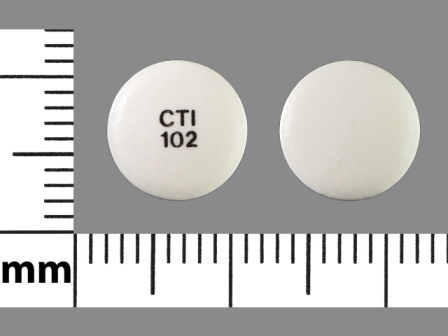
- NDC: *
- 42291-230
- Imprint:
- CTI 102
- Color:
- White
- Shape:
- Round
- Size:
- 9 mm
- Segments:
- 1
- Drug:
- Diclofenac
- Labeler: *
- Avkare, Inc.
- Category:
- Anti-Inflammatory
Source: US National Library of Medicine (NLM)
- NDC: *
- 60429-057
- Imprint:
- CTI 102
- Color:
- White
- Shape:
- Round
- Size:
- 9 mm
- Segments:
- 1
- Drug:
- Diclofenac
- Labeler: *
- Golden State Medical Supply, Inc.
- Category:
- Anti-Inflammatory
Source: US National Library of Medicine (NLM)
Took this pill to help treat diabetic neuropothy. It caused severe stomach ache and dry mouth for me! ! Good luck....hope it helps you. ## Hello, Beege! How are you? I'm very sorry about the experience you had. This tablet is listed by the national library of medicine as containing 50mgs of Diclofenac, which is classified by the FDA as a nonsteroidal anti-inflammatory. They are most commonly used to treat pain, fever and swelling. Stomach irritation and dry mouth can be common side effects, along with nausea, dizziness and headache. Are there any questions or concerns?
Yes the pill is very small and is white on one side it has the letters on it are cti and the number under it is 102? ## Hi, Nick! How are you? This tablet contains 50mgs of Diclofenac in a time release formulation, it is a nonsteroidal anti-inflammatory that's used to treat pain, fever and swelling. Side effects may include nausea, dizziness, headache and stomach irritation. Learn more Diclofenac details here. Is there anything else I can help with?
Diclofenac Sodium 50 mg Delayed Release Tablet by Carlsbad Technology, Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 61442-102-01, 61442-102-10, 61442-102-60 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium Delayed Release 75 mg Oral Tablet, Delayed Release by Repharm Pharmaceuticals ## Markings: CTI;103 ## Shape: Round ## Color: White ## Package Codes: 61145-102-06, 61145-102-12, 61145-102-18 ## Active Ingredients: Diclofenac Sodium
Lovastatin 40 mg Oral Tablet by Bryant Ranch Prepack ## Markings: CTI;143 ## Shape: Round ## Color: White ## Package Codes: 63629-1020-0, 63629-1020-1, 63629-1020-2, 63629-1020-3, 63629-1020-5, 63629-1020-6, 63629-1020-8
Diclofenac Sodium Delayed Release 50 mg Oral Tablet, Delayed Release by Unit Dose Services ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 50436-1022-1 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium 50 mg Delayed Release Tablet by Avkare, Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 42291-230-10, 42291-230-18 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium D/R 50 mg Oral Tablet, Delayed Release by Direct Rx ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 61919-074-30, 61919-074-60, 61919-074-90 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium D/R 75 mg Oral Tablet, Delayed Release by Direct Rx ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 61919-075-20, 61919-075-30, 61919-075-60, 61919-075-90 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium 50 mg Oral Tablet, Delayed Release by Blenheim Pharmacal, Inc. ## Markings: CTI;102; ## Shape: Round ## Color: White ## Package Codes: 10544-188-21, 10544-188-30, 10544-188-60, 10544-188-90 ## Active Ingredients: Diclofenac Sodium
Lidovix Delayed ReleaseLidovix Oral; Topical Kit by Primary Pharmaceuticals, Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 72275-708-77 ## Active Ingredients:
Diclofenac Sodium 50 mg Oral Tablet, Delayed Release by Preferred Pharmaceuticals Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 68788-8952-1, 68788-8952-3, 68788-8952-6, 68788-8952-8, 68788-8952-9 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium Delayed Release 50 mg Oral Tablet, Delayed Release by Proficient Rx Lp ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 71205-995-00, 71205-995-11, 71205-995-30, 71205-995-60, 71205-995-72, 71205-995-78, 71205-995-90 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium Delayed Release 50 mg Oral Tablet, Delayed Release by Remedyrepack Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 52125-372-02, 52125-372-19 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium 50 mg Oral Tablet, Delayed Release by Golden State Medical Supply, Inc. ## Markings: CTI;102; ## Shape: Round ## Color: White ## Package Codes: 60429-057-01, 60429-057-10, 60429-057-18, 60429-057-60 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium Delayed Release 50 mg Oral Tablet, Delayed Release by Remedyrepack Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 70518-0756-0 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium Delayed Release 50 mg Oral Tablet, Delayed Release by Remedyrepack Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 70518-2707-0, 70518-2707-1, 70518-2707-2 ## Active Ingredients: Diclofenac Sodium
Diclofenac Sodium 50 mg Delayed Release Tablet by Aphena Pharma Solutions - Tennessee, Inc. ## Markings: CTI;102 ## Shape: Round ## Color: White ## Package Codes: 43353-784-53 ## Active Ingredients: Diclofenac Sodium