61919-773 : Omeprazole Dr 40 mg/1 Oral Capsule, Delayed Release
| NDC: | 61919-773 |
| Labeler: | Direct Rx |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Omeprazole Dr Omeprazole Dr |
| Dosage Form: | Oral Capsule, Delayed Release |
| Application #: | ANDA076048 |
| Rev. Date: |
Appearance:
| Markings: | APO;040 |
| Shapes: |
Capsule |
| Colors: |
 Pink / Pink /
 Brown Brown |
| Size (mm): | 21 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
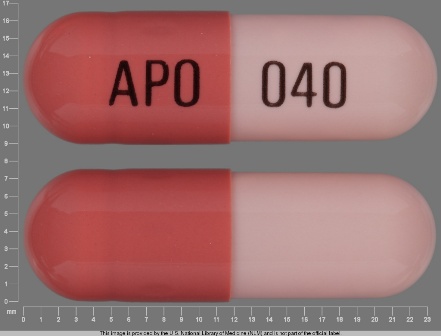
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 61919-773-30: 30 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (61919‑773‑30)
- 61919-773-60: 60 BOTTLE IN 1 BOTTLE (61919‑773‑60) > 30 CAPSULE, DELAYED RELEASE IN 1 BOTTLE (61919‑773‑30)
Active Ingredients:
- Omeprazole
Dosage Strength:
- 40 mg
Inactive Ingredients:
- Magnesium Hydroxide
- Mannitol
- Methacrylic Acid - Ethyl Acrylate Copolymer (1:1) Type a
- Povidone K30
- Triethyl Citrate
- Gelatin
- Ferric Oxide Red
- Titanium Dioxide
- Ammonia
- Ferrosoferric Oxide
- Alcohol
- Isopropyl Alcohol
- Butyl Alcohol
- Potassium Hydroxide
- Propylene Glycol
- Shellac
Pharmaceutical Classes:
- Proton Pump Inhibitor [EPC]
- Proton Pump Inhibitors [MoA]
- Cytochrome P450 2C19 Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 61919-176 Omeprazole Dr 20 mg/1 Oral Capsule, Delayed Release by Direct Rx
- 61919-807 Omeprazole Dr 20 mg Oral Capsule, Delayed Release by Direxctrx
- 51655-022 Omeprazole 20 mg Oral Capsule, Delayed Release by Northwind Pharmaceuticals, LLC
- 80425-0070 Omeprazole Dr 20 mg Oral Capsule, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0071 Omeprazole Dr 20 mg Oral Capsule, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0109 Omeprazole Dr 40 mg Oral Capsule, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
- 80425-0169 Omeprazole Dr 40 mg Oral Capsule, Delayed Release by Advanced Rx Pharmacy of Tennessee, LLC
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 61919-772Next: 61919-774 >
Related Discussions:
Omeprazole vs Pantoprazole
advantages of omeprazole over pantoprazole ## There really is no benefit to using one over the other, they both work the... 19 replies
advantages of omeprazole over pantoprazole ## There really is no benefit to using one over the other, they both work the... 19 replies
Omeprazole Dr 40mg Side Effects
I have only taken this pill twice and I am shaky, nauseous and loss of appetite. I am hoping it is because i am taking t... 13 replies
I have only taken this pill twice and I am shaky, nauseous and loss of appetite. I am hoping it is because i am taking t... 13 replies
Omeprazole Capsules 20 Mg
Please let me know how Omeprazole Ip 20 Mg capsules are supposed to be administerred? ## Omeprazole is a proton pump inh... 13 replies
Please let me know how Omeprazole Ip 20 Mg capsules are supposed to be administerred? ## Omeprazole is a proton pump inh... 13 replies
omeprazole 20mg
pink/reddish-brown oblong shaped capsule half of the capsule has:APO and the other half has:020 ## Just to clarify, I lo... 7 replies
pink/reddish-brown oblong shaped capsule half of the capsule has:APO and the other half has:020 ## Just to clarify, I lo... 7 replies
Omeprazole Manufacturers changed - new meds causing pain in chest!
The pharmacy has changed the manufacturer of my 20 mg Omeprazole from Sandoz to Dr.Reddy Labs. Know when I take my dosag... 6 replies
The pharmacy has changed the manufacturer of my 20 mg Omeprazole from Sandoz to Dr.Reddy Labs. Know when I take my dosag... 6 replies
Omeprazole Cr
Does this medicine cause you to have a strong odor in your urine? ## No, I don't see it listed anywhere as a possibl... 5 replies
Does this medicine cause you to have a strong odor in your urine? ## No, I don't see it listed anywhere as a possibl... 5 replies
Omeprazole Dr 40mg
The capsule is green on one side, blue on the other and contains 40 mgs of Omeprazole. I have been taking it for two wee... 5 replies
The capsule is green on one side, blue on the other and contains 40 mgs of Omeprazole. I have been taking it for two wee... 5 replies
Omeprazole 40mg
Is Omeprazole gluten free? I have questions about the medication and the casing... ## Without knowing the specific manuf... 5 replies
Is Omeprazole gluten free? I have questions about the medication and the casing... ## Without knowing the specific manuf... 5 replies
Omeprazole Dr
was the color of this capsule change from aqua mylan6150 to ku 118 white/brown ## Mylan 6150 is manufactured by Mylan Ph... 4 replies
was the color of this capsule change from aqua mylan6150 to ku 118 white/brown ## Mylan 6150 is manufactured by Mylan Ph... 4 replies
Omeprazole + Salamol plus email addresscorrection
I have just been prescribed Omeprazole for suspected acid reflux. As I am still breathless from previous bronchitis, can... 4 replies
I have just been prescribed Omeprazole for suspected acid reflux. As I am still breathless from previous bronchitis, can... 4 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.