60687-332 : Ramipril 2.5 mg Oral Capsule
| NDC: | 60687-332 |
| Labeler: | American Health Packaging |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Ramipril Ramipril |
| Dosage Form: | Oral Capsule |
| Application #: | ANDA078832 |
| Rev. Date: |
Appearance:
| Markings: | ZA;44;2;5mg |
| Shapes: |
Capsule |
| Colors: |
 White / White /
 Orange Orange |
| Size (mm): | 14 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
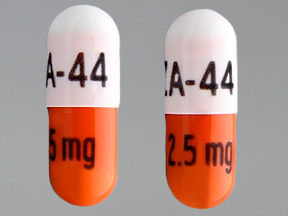
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 60687-332-01: 100 BLISTER PACK IN 1 BOX, UNIT‑DOSE (60687‑332‑01) > 1 CAPSULE IN 1 BLISTER PACK (60687‑332‑11)
Active Ingredients:
- Ramipril
Dosage Strength:
- 2.5 mg
Inactive Ingredients:
- Gelatin, Unspecified
- Starch, Corn
- Titanium Dioxide
- D&c Yellow No. 10
- Fd&c Red No. 40
Pharmaceutical Classes:
- Angiotensin Converting Enzyme Inhibitor [EPC]
- Angiotensin-converting Enzyme Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 60687-321 Ramipril 1.25 mg Oral Capsule by American Health Packaging
- 60687-343 Ramipril 5 mg Oral Capsule by American Health Packaging
- 60687-354 Ramipril 10 mg Oral Capsule by American Health Packaging
- 68084-266 Ramipril 2.5 mg Oral Capsule by American Health Packaging
- 68084-267 Ramipril 5 mg Oral Capsule by American Health Packaging
- 68084-268 Ramipril 10 mg Oral Capsule by American Health Packaging
- 68084-294 Ramipril 1.25 mg Oral Capsule by American Health Packaging
- 0054-0106 Ramipril 1.25 mg Oral Capsule by Roxane Laboratories, Inc
- 0054-0107 Ramipril 2.5 mg Oral Capsule by Roxane Laboratories, Inc
- 0054-0108 Ramipril 5 mg Oral Capsule by Roxane Laboratories, Inc
- 0054-0109 Ramipril 10 mg Oral Capsule by Roxane Laboratories, Inc
- 0093-7436 Ramipril 2.5 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7437 Ramipril 5 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 0093-7438 Ramipril 10 mg Oral Capsule by Teva Pharmaceuticals USA Inc
- 10544-588 Ramipril 10 mg Oral Capsule by Blenheim Pharmacal, Inc.
- 16252-570 Ramipril 1.25 mg Oral Capsule by Cobalt Laboratories
- 16252-571 Ramipril 2.5 mg Oral Capsule by Cobalt Laboratories
- 16252-572 Ramipril 5 mg Oral Capsule by Cobalt Laboratories
- 16252-573 Ramipril 10 mg Oral Capsule by Cobalt Laboratories
- 16729-152 Ramipril 1.25 mg Oral Capsule by Accord Healthcare Inc.
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 60687-330Next: 60687-333 >
Related Discussions:
Ramipril & Amlodipine
I have had high BP for some time, treated with 10 mg Ramipril. The readings have always remained on the high side. Today... 13 replies
I have had high BP for some time, treated with 10 mg Ramipril. The readings have always remained on the high side. Today... 13 replies
Ramipril Blue & White Capsule 10 Mg NOW Purple & White
Have been taking Ramipril (Altace) 10mg for probably 10 years and they have ALWAYS been white and BLUE capsules. Last we... 8 replies
Have been taking Ramipril (Altace) 10mg for probably 10 years and they have ALWAYS been white and BLUE capsules. Last we... 8 replies
ramipril cause clogging at back of throat
I have been on ramipril for a few months now to go with my amlodipine 5mg for high blood pressure. I am mainly at night ... 7 replies
I have been on ramipril for a few months now to go with my amlodipine 5mg for high blood pressure. I am mainly at night ... 7 replies
Ramipril weight gain side effects
I have been taking Ramipril for two weeks and I am wondering if weight gain is one of the side effects. Finally, after b... 4 replies
I have been taking Ramipril for two weeks and I am wondering if weight gain is one of the side effects. Finally, after b... 4 replies
Ramipril Side Effects
i have been on 12.5mg of ramipril each day for the past 2 and half years. We have now got a new doctor in our practice a... 3 replies
i have been on 12.5mg of ramipril each day for the past 2 and half years. We have now got a new doctor in our practice a... 3 replies
Ramipril Changed
They changed my generic Ramipril to another with markings RDY 441. I was on generic Ramipril ( called Colbolt ) and it w... 3 replies
They changed my generic Ramipril to another with markings RDY 441. I was on generic Ramipril ( called Colbolt ) and it w... 3 replies
Ramipril and Kidney Function
I had my kidney function tested in February and it was 85. I then started taking Ramipril & by November it had dropp... 2 replies
I had my kidney function tested in February and it was 85. I then started taking Ramipril & by November it had dropp... 2 replies
ramipril and nabivelol can both be used for hypertention
Can I both nabivelol and ramipril taken as sos when hypertention is uncontrol? ## Hello, Narinder! How are you doing? Ye... 2 replies
Can I both nabivelol and ramipril taken as sos when hypertention is uncontrol? ## Hello, Narinder! How are you doing? Ye... 2 replies
Ramipril Tablets IP Hopace 5
for which disease is it used ## Ramipril is an ACE inhibitor that is most commonly used to treat high blood pressure and... 2 replies
for which disease is it used ## Ramipril is an ACE inhibitor that is most commonly used to treat high blood pressure and... 2 replies
Ramipril and coversyl plus
What is the coversyl plus 4 mg equivalent in Cardace(Ramipril) ## is coversyl 4mg the same as ramipril 10 mg ## my fathe... 2 replies
What is the coversyl plus 4 mg equivalent in Cardace(Ramipril) ## is coversyl 4mg the same as ramipril 10 mg ## my fathe... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.