55154-3526 : Perphenazine 2 mg Oral Tablet
| NDC: | 55154-3526 |
| Labeler: | Cardinal Health |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Perphenazine Perphenazine |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA089683 |
| Rev. Date: |
Appearance:
| Markings: | GG18 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 6 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
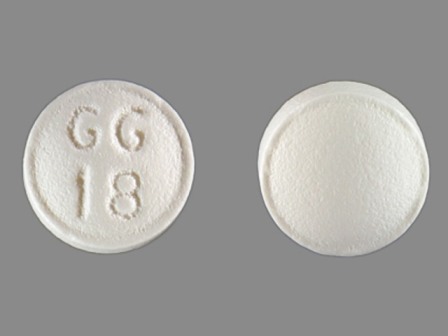
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 55154-3526-0: 10 BLISTER PACK IN 1 BAG (55154‑3526‑0) > 1 TABLET, FILM COATED IN 1 BLISTER PACK
Active Ingredients:
- Perphenazine
Dosage Strength:
- 2 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Hydroxypropyl Cellulose
- Hypromelloses
- Hypromellose 2910 (3 Mpa.s)
- Lactose Monohydrate
- Magnesium Stearate
- Polyethylene Glycols
- Polysorbate 80
- Starch, Corn
- Titanium Dioxide
Pharmaceutical Classes:
- Phenothiazine [EPC]
- Phenothiazines [Chemical/Ingredient]
Related Products:
Based on records with the same trade name.- 0378-5350 Perphenazine 2 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0378-5351 Perphenazine 4 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0378-5352 Perphenazine 8 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0378-5353 Perphenazine 16 mg Oral Tablet, Film Coated by Mylan Pharmaceuticals Inc.
- 0591-4101 Perphenazine 2 mg Oral Tablet, Film Coated by Actavis Pharma, Inc.
- 0591-4102 Perphenazine 4 mg Oral Tablet, Film Coated by Actavis Pharma, Inc.
- 0591-4103 Perphenazine 8 mg Oral Tablet, Film Coated by Actavis Pharma, Inc.
- 0591-4104 Perphenazine 16 mg Oral Tablet, Film Coated by Actavis Pharma, Inc.
- 0603-5060 Perphenazine 2 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5061 Perphenazine 4 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5062 Perphenazine 8 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5063 Perphenazine 16 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5090 Perphenazine 2 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5091 Perphenazine 4 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5092 Perphenazine 8 mg Oral Tablet by Qualitest Pharmaceuticals
- 0603-5093 Perphenazine 16 mg Oral Tablet by Qualitest Pharmaceuticals
- 0615-3584 Perphenazine 2 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-3585 Perphenazine 4 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0615-4511 Perphenazine 8 mg Oral Tablet by Ncs Healthcare of Ky, Inc Dba Vangard Labs
- 0781-1046 Perphenazine 2 mg Oral Tablet by Sandoz Inc
- More related products ...
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 55154-3525Next: 55154-3528 >
Related Discussions:
Is Perphenazine-Amitriptyline a sleep Med?
Is this a sleep medication? Any information would be helpful. ## would it be safe for a 15 year old ## what is perphen/a... 4 replies
Is this a sleep medication? Any information would be helpful. ## would it be safe for a 15 year old ## what is perphen/a... 4 replies
Paxil CR and Perphenazine
i am taking one in the day and one in the night dont feel good and confused and sick with diarrhea and constipation ## I... 3 replies
i am taking one in the day and one in the night dont feel good and confused and sick with diarrhea and constipation ## I... 3 replies
withdraw from perphenazine/amitriptyline
My aunt was taken to the hospital and the doctor in the emergency room stopped her perphenazine/amitriptyline after taki... 1 reply
My aunt was taken to the hospital and the doctor in the emergency room stopped her perphenazine/amitriptyline after taki... 1 reply
Substitute for Perphenazine?
My wife ran out of this pill. She was taking two at bed time. Now she is having bad dreams and says her body hurts all o... 4 replies
My wife ran out of this pill. She was taking two at bed time. Now she is having bad dreams and says her body hurts all o... 4 replies
amox clav and amitryptline with perphenazine
I am taking amox clav. can I take amitruptaline with perphenazine also ## Hello, Joyce! How are you? I didn't find a... 1 reply
I am taking amox clav. can I take amitruptaline with perphenazine also ## Hello, Joyce! How are you? I didn't find a... 1 reply
perphenazine/amitriptyline
I think it is a generic for Trivil or Tryvil...
I think it is a generic for Trivil or Tryvil...
perphenazine
trilafon...
trilafon...
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.