50436-6660 : Digox 0.25 mg Oral Tablet
| NDC: | 50436-6660 |
| Labeler: | Unit Dose Services |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Digox Digox |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA076268 |
| Rev. Date: |
Appearance:
| Markings: | JSP;545 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 7 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
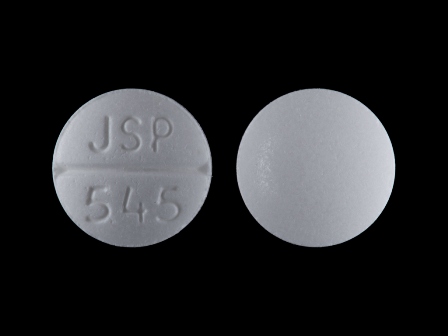
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 50436-6660-1: 30 TABLET IN 1 BOTTLE (50436‑6660‑1)
Active Ingredients:
- Digoxin
Dosage Strength:
- 250 ug/1
Inactive Ingredients:
- Silicon Dioxide
- Croscarmellose Sodium
- Anhydrous Lactose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Stearic Acid
Pharmaceutical Classes:
- Cardiac Glycoside [EPC]
- Cardiac Glycosides [CS]
Related Products:
Based on records with the same trade name.- 50436-0324 Digox 0.25 mg Oral Tablet by Unit Dose Services
- 0527-1324 Digox 0.125 mg Oral Tablet by Lannett Company, Inc.
- 0527-1325 Digox 0.25 mg Oral Tablet by Lannett Company, Inc.
- 24236-454 Digox 250 ug/1 Oral Tablet by Remedyrepack Inc.
- 49349-797 Digox 0.25 mg Oral Tablet by Remedyrepack Inc.
- 49999-180 Digoxin 125 Mcg Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 49999-181 Digox 250 ug/1 Oral Tablet by Lake Erie Medical Dba Quality Care Products LLC
- 53217-005 Digox 250 ug/1 Oral Tablet by Aidarex Pharmaceuticals LLC
- 55289-626 Digox 0.25 mg Oral Tablet by Pd-rx Pharmaceuticals, Inc.
- 60760-003 Digox 0.125 mg Oral Tablet by St Marys Medical Park Pharmacy
- 60760-406 Digox 250 ug/1 Oral Tablet by St Marys Medical Park Pharmacy
- 63187-740 Digox 125 ug/1 Oral Tablet by Proficient Rx Lp
- 64725-1324 Digox 125 ug/1 Oral Tablet by Tya Pharmaceuticals
- 68151-1602 Digox 125 ug/1 Oral Tablet by Carilion Materials Management
- 68151-1603 Digox 250 ug/1 Oral Tablet by Carilion Materials Management
- 76237-294 Digox 0.125 mg Oral Tablet by Mckesson Contract Packaging
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 50436-6652Next: 50436-6664 >
Related Discussions:
Digoxin for a-fib
Dr. Prescribing this for a-fib. I have read that there is a high mortality rate for a-fib on this. Is there anything out... 3 replies
Dr. Prescribing this for a-fib. I have read that there is a high mortality rate for a-fib on this. Is there anything out... 3 replies
digoxin side effects
I am taking Cartia xt 120mg, but now the doctor added Digoxin and after a 1/2 hour I started with PVCs. Could there be i... 3 replies
I am taking Cartia xt 120mg, but now the doctor added Digoxin and after a 1/2 hour I started with PVCs. Could there be i... 3 replies
Digoxin Brand Global
I have been taking Digoxin .125 for afib for 3 years with no problems. A few weeks ago I started feeling tired.. running... 1 reply
I have been taking Digoxin .125 for afib for 3 years with no problems. A few weeks ago I started feeling tired.. running... 1 reply
digoxin toxicity
What causes overdose of this medication and the symptoms? Is it detected by blood work? ## According to the National Ins... 1 reply
What causes overdose of this medication and the symptoms? Is it detected by blood work? ## According to the National Ins... 1 reply
Digoxin 981 versus JSP 544
I just renewed my Digoxin 125 MCG prescription. I have been getting JSP 544 and just received 981. The size of the 981 i... 1 reply
I just renewed my Digoxin 125 MCG prescription. I have been getting JSP 544 and just received 981. The size of the 981 i... 1 reply
number on digoxin 125 mg heart tablets
I need to know what the digoxin 125 ng pill looks like. My husband messed up his meds and I want to make sure which is d... 1 reply
I need to know what the digoxin 125 ng pill looks like. My husband messed up his meds and I want to make sure which is d... 1 reply
Femara and Digoxin - do they mix?
I am taking letrozole and have now been put on digoxin are there any known side effects. I need to take these as I have ... 1 reply
I am taking letrozole and have now been put on digoxin are there any known side effects. I need to take these as I have ... 1 reply
Can Lanoxin (Digoxin) and Vastarel (Trimetazidine) be taken together?
I am taking Lanoxin and Aspirin (2700mg) a day for Rheumatic Heart Disease. Is it safe to take Vastarel together with th... 3 replies
I am taking Lanoxin and Aspirin (2700mg) a day for Rheumatic Heart Disease. Is it safe to take Vastarel together with th... 3 replies
Overdose of Eliquis, Digoxin and Sotalol
I accidentally took my meds twice this morning. It was 5 mg eliquis, 125 mg digoxin and 40 mg sotalol. My question is sh... 1 reply
I accidentally took my meds twice this morning. It was 5 mg eliquis, 125 mg digoxin and 40 mg sotalol. My question is sh... 1 reply
doxy cycl hycl 100mg and digoxin interaction
I have been taking digoxin since January '14. Now I have a skin infection and have been prescribed Doxycycl Hycl 100... 1 reply
I have been taking digoxin since January '14. Now I have a skin infection and have been prescribed Doxycycl Hycl 100... 1 reply
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.