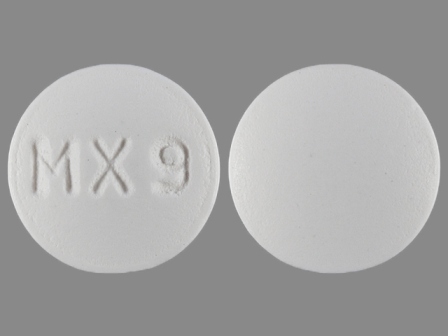
Uceris
Active Ingredient(s): BudesonideFDA Approved: * January 14, 2013
Pharm Company: * SANTARUS
Category: Anti-Inflammatory
Budesonide (BUD), sold under the brand name Pulmicort among others, is a medication of the corticosteroid type.[3] It is available as an inhaler, nebulization solution, pill, nasal spray, and rectal forms.[3][4] The inhaled form is used in the long-term management of asthma and chronic obstructive pulmonary disease (COPD).[3][5][6] The nasal spray is used for allergic rhinitis and nasal polyps.&... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.