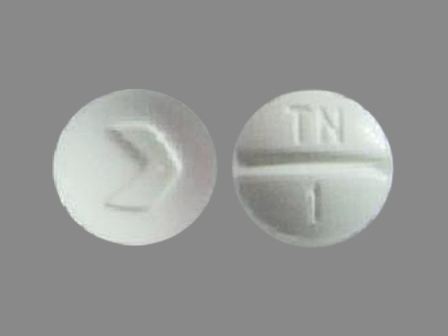
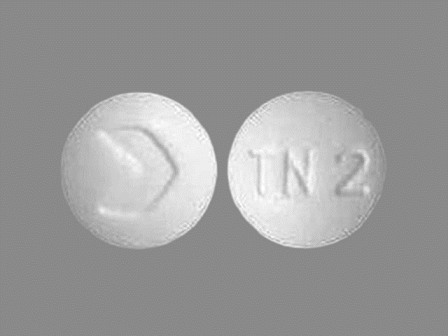
Trandolapril
FDA Approved: * December 12, 2006Pharm Company: * TEVA PHARMS
Category: Blood Pressure
Trandolapril is an ACE inhibitor used to treat high blood pressure. It may also be used to treat other conditions. It is similar in structure to another ACE Inhibitor, Ramipril but has a cyclohexane group. It also is a pro-drug and must get metabolized. It has an extended half-life and therefore has a higher potency. It was patented in 1981, and approved for medical use in 1993.[1] It is marketed by Abbott Laboratories under the brand name Mavik. Contents 1 Side effects 2 ... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.3 Discussions
Dosage List