Pancreaze
Active Ingredient(s): PancrelipaseFDA Approved: * April 12, 2010
Pharm Company: * ORTHO MCNEIL JANSSEN
Category: Enzymes
An amylase (/æmlez/) is an enzyme that catalyses the hydrolysis of starch (Latin amylum) into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolys... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.1 Discussion
Dosage List
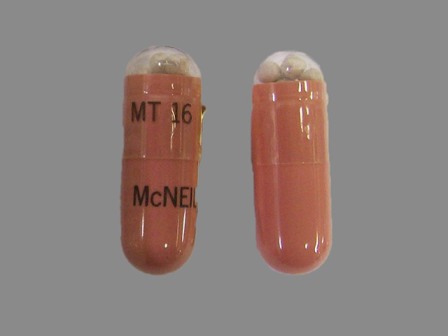
Pancreaze (Pancrelipase Lipase 16800 [usp'u] / Pancrelipase Amylase 70000 [usp'u] / Pancrelipase Protease 40000 [usp'u])
NDC: 50458-343
Labeler:
Janssen Pharmaceuticals, Inc.
Pancreaze (Pancrelipase Lipase 4200 [usp'u] / Pancrelipase Amylase 17500 [usp'u] / Pancrelipase Protease 10000 [usp'u])
NDC: 50458-341
Labeler:
Janssen Pharmaceuticals, Inc.
Pancreaze (Pancrelipase Lipase 10500 [usp'u] / Pancrelipase Amylase 43750 [usp'u] / Pancrelipase Protease 25000 [usp'u])
NDC: 50458-342
Labeler:
Janssen Pharmaceuticals, Inc.
Pancreaze (Pancrelipase Lipase 21000 [usp'u] / Pancrelipase Amylase 61000 [usp'u] / Pancrelipase Protease 37000 [usp'u])
NDC: 50458-346
Labeler:
Janssen Pharmaceuticals, Inc.