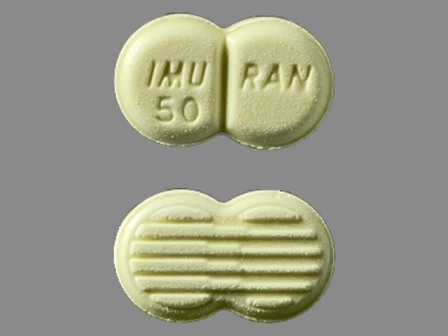
Imuran
Active Ingredient(s): AzathioprineFDA Approved: * March 20, 1968
Pharm Company: * PROMETHEUS LABS
Category: Immunosuppressive
Azathioprine (AZA), sold under the brand name Imuran, among others, is an immunosuppressive medication.[2] It is used in rheumatoid arthritis, granulomatosis with polyangiitis, Crohn's disease, ulcerative colitis, and systemic lupus erythematosus, and in kidney transplants to prevent rejection.[2][3][4][5] It is taken by mouth or injected into a vein.[2] Common side effects include bone-marrow supp... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.3 Discussions
Dosage List
Related Brands
Drugs with the same active ingredientsPopular Topics
My father has been a patient of myasthenia gravis for years & is also diabetic. Is imuran safe for him? He has been ...
I have read that Imuran can cause brain abscesses. Has anyone got knowledge if this is so?...
I was diagnosed with Pnemoititis(extreme) I have been on Imuran for appx 9mo's. Lungs are showing improvement. Do ha...