Edarbi
Active Ingredient(s): Azilsartan MedoxomilFDA Approved: * February 25, 2011
Pharm Company: * TAKEDA PHARMS NA
Category: Blood Pressure
Azilsartan is an angiotensin II receptor antagonist used in the treatment of hypertension,[1][2][3] developed by Takeda. It is marketed in tablet form under the brand name Edarbi as the prodrug azilsartan medoxomil.[4] The most common adverse reaction in adults is diarrhea.[1] It is also sold as a combination drug with chlortalidone under the brand name Edarbyclor.[5] Contents 1 Structure activit... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.5 Discussions
Dosage List

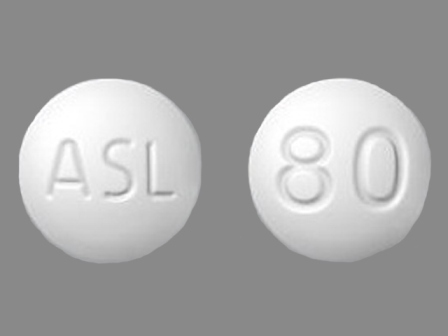
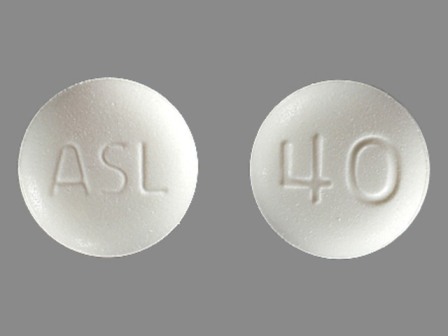
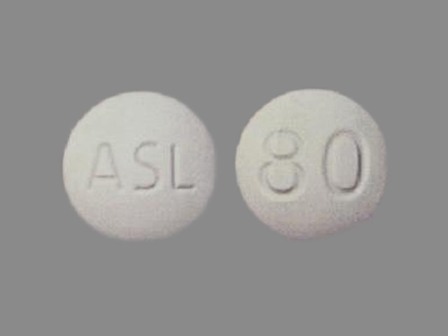
Popular Topics
What time of day should I take Edarbi? ## What has your doctor advised? Edarbi is most commonly used to help treat high ...
I have only been taking edarbi by cutting it in half, been taking it for a month...last reading 126/78 ## How much has y...
Has anyone had burning of hands and feet and callouses on hands and feet from edarbi? ## Edarbi (Azilsartan Medoxomil) d...