Amturnide
Active Ingredient(s): Amlodipine + Aliskiren + HydrochlorothiazideFDA Approved: * December 21, 2010
Pharm Company: * NOVARTIS PHARMS
Category: Blood Pressure
Aliskiren/amlodipine/hydrochlorothiazide, sold under the brand name Amturnide, is a fixed-dose combination medication that is used to treat high blood pressure.[1][2] It contains aliskiren, amlodipine, and hydrochlorothiazide.[1] It is taken by mouth.[1] It was approved for medical use in the United States in December 2010.[1][3][4] Amturnide was withdrawn by Novartis from the US ma... [wikipedia]
* May have multiple approval dates, manufacturers, or labelers.Dosage List
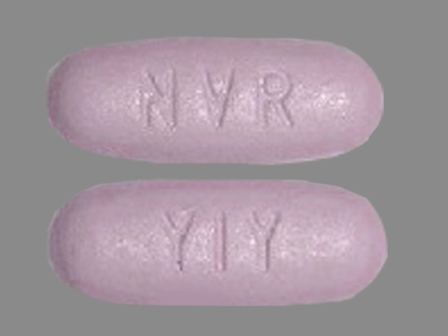
Amturnide (Aliskiren Hemifumarate 165.8 mg / Amlodipine Besylate 6.9 mg / Hctz 12.5 mg) Oral Tablet
NDC: 0078-0610
Labeler:
Novartis Pharmaceuticals Corporation
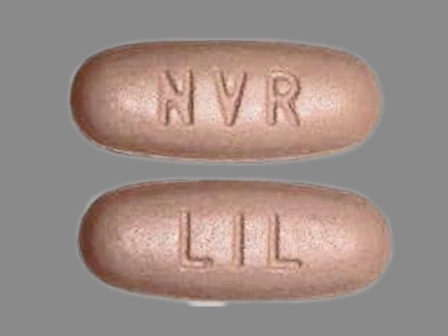
Amturnide (Aliskiren Hemifumarate 331.5 mg / Amlodipine Besylate 6.9 mg / Hctz 12.5 mg) Oral Tablet
NDC: 0078-0611
Labeler:
Novartis Pharmaceuticals Corporation
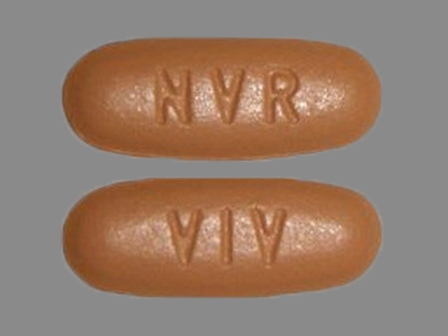
Amturnide (Aliskiren Hemifumarate 331.5 mg / Amlodipine Besylate 13.9 mg / Hctz 25 mg) Oral Tablet
NDC: 0078-0614
Labeler:
Novartis Pharmaceuticals Corporation
Amturnide (Aliskiren Hemifumarate 331.5 mg / Amlodipine Besylate 6.9 mg / Hctz 25 mg) Oral Tablet
NDC: 0078-0612
Labeler:
Novartis Pharmaceuticals Corporation
Amturnide (Aliskiren Hemifumarate 331.5 mg / Amlodipine Besylate 13.9 mg / Hctz 12.5 mg) Oral Tablet
NDC: 0078-0613
Labeler:
Novartis Pharmaceuticals Corporation